It's pretty hard to imagine the world through someone else's eyes, especially different animals. But a new study using lab-grown human retinas reveals that even between different humans, our vision is extremely diverse.
And it might be to do with how the red and green cones form in our retinas. Cones are light-sensing cells in vertebrates' eyes; their combined responses to different wavelengths enable color vision.
Humans and some closely related primates are some of the only mammals known that can see the color red, as well as green and blue.
Other animals can also see red, like many birds and some insects. The kind of vision an animal has is closely related to its evolution alongside plants that produce fruits and flowers. This ability has been pretty useful, for instance, for spotting a ripe red apple among a dense canopy of green.
Another mammal outlier with the ability to see red is the honey possum (Tarsipes rostratus). This Australian marsupial pollinator has a bird-like ability to probe the nectar from a blushing banksia, in a fascinating example of convergent evolution.
Our red and green cones are basically identical, with slightly different chemistry to determine which color they'll detect. A protein called opsin comes in two different 'flavors', red-sensitive or green-sensitive, and their genetic 'recipes' sit side-by-side on the X chromosome.
So it's very easy for them to get mixed up in recombination, resulting in variations of congenital red-green color blindness.
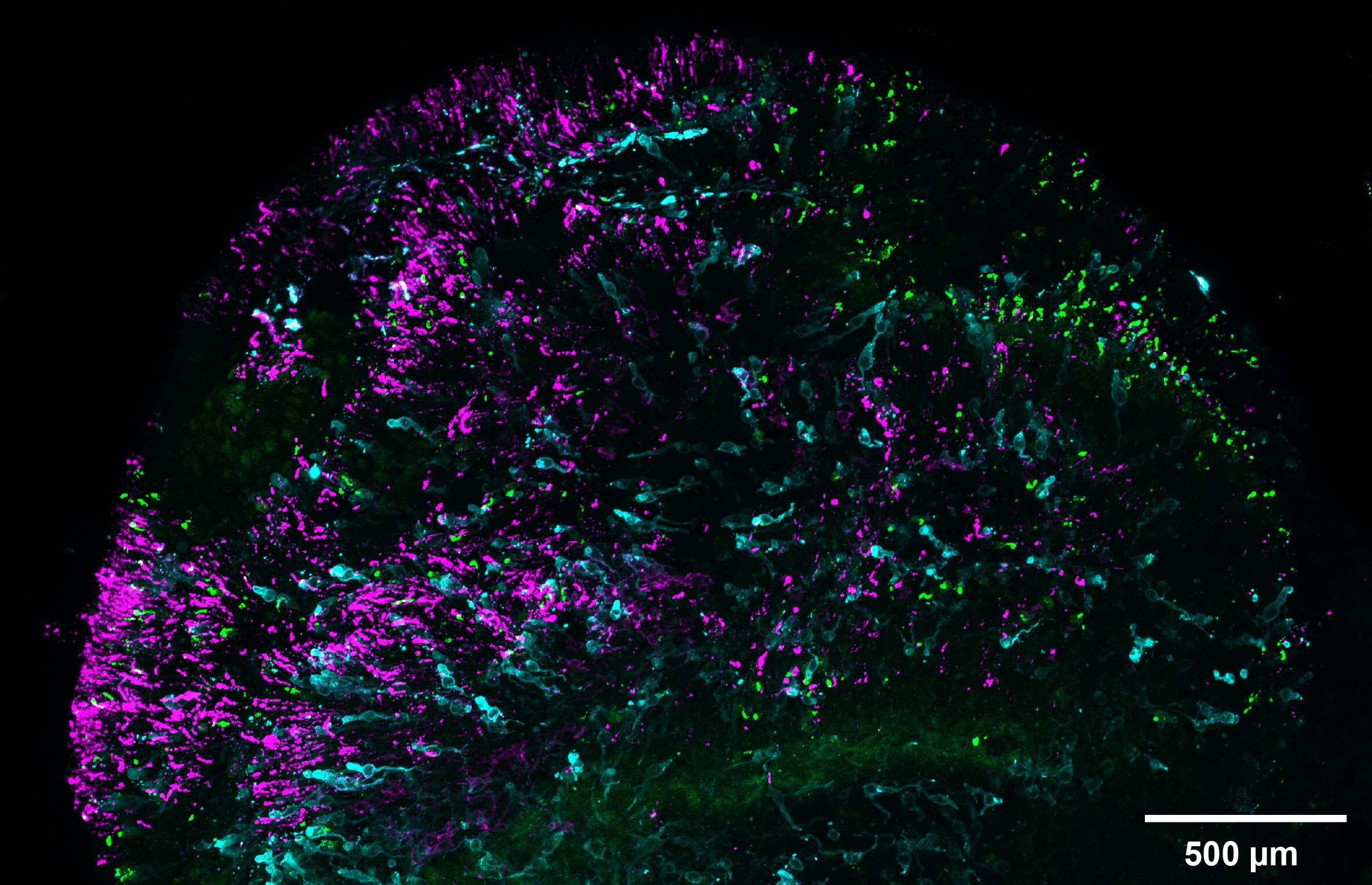
Now, new research offers some clarity as to what those key vision-defining ingredients – making up just a 4 percent difference between the genes that code for these proteins – actually are.
We previously thought cone determination was basically random, though more recent studies have pointed to thyroid levels playing a role.
But a team from Johns Hopkins University and the University of Washington has discovered that levels of a molecule derived from vitamin A called retinoic acid make or break red-green cone ratios, at least in the case of their lab-grown retinas.
"These retinal organoids allowed us for the first time to study this very human-specific trait," says developmental biologist Robert Johnston from Johns Hopkins University. "It's a huge question about what makes us human, what makes us different."
In the lab, retinas exposed to more retinoic acid during early development (the first 60 days) resulted in higher ratios of green cones across the organoid after 200 days, while immature cones exposed to low levels of the acid developed into red cones later on.
The timing matters, too. If the retinoic acid was introduced at 130 days onwards, the effect was the same as if none had been added at all. This suggests the acid determines cone type early, and can't cause red cones to 'switch' into green cones that have already matured.
All the lab-grown retinas had similar cone densities, which allowed the team to rule out cone cell death as affecting the ratio of red to green.
Developmental biologist Sarah Hadyniak, who co-authored the study while at Johns Hopkins University, says their findings have implications for figuring out exactly how retinoic acid is acting on genes.
To get a sense of how much this could be affecting human vision, the researchers studied the retinas of 738 male adults with no signs of color vision deficiency.
The researchers were astonished at the natural variation in red/green cone ratio across this group.
"Seeing how the green and red cone proportions changed in humans was one of the most surprising findings of the new research", Hadyniak says.
It's unclear how this much variation could occur without affecting changes in vision. As Johnston put it, "if these types of cells determined the length of a human arm, the different ratios would produce amazingly different arm lengths."
This research was published in PLOS Biology.
