The World Health Organization has approved a new vaccine that scientists argue will be a game-changer in the fight against malaria, which kills half a million people in Africa every year.
Trials have shown that the R21/Matrix vaccine, developed by Oxford University together with the Serum Institute of India, reduces malaria by up to 75 percent. It can be manufactured cheaply and on a mass scale.
The Conversation Weekly spoke to chief investigator Adrian Hill, who is also director of the Jenner Institute at the University of Oxford, about this revolutionary vaccine. Below are edited excerpts from the podcast.
Why is the R21/Matrix vaccine a game-changer?
We're seeing about 75 percent efficacy by counting the reduction in numbers of malaria episodes over a year. The best vaccine prior to this was about 50 percent over a year, and lower than that over three years.
This is a material improvement, but that's not the main improvement. The big difference is how you can manufacture it at a scale that is really needed to protect most of the children who need a malaria vaccine in Africa.
There are about 40 million children born every year in malaria areas in Africa who would benefit from a vaccine. Ours is a four-dose vaccine over 14 months, so you need about 160 million doses. We can achieve that.
The Serum Institute of India, our manufacturing and commercial partner, can produce hundreds of millions of doses of this vaccine each year, whereas the previous vaccine could be manufactured at a scale of six million doses a year from 2023 to 2026, according to Unicef reports.
The third real advantage of this vaccine is its cost. We were well aware that we couldn't produce a US$100 vaccine. It wouldn't fly for international agencies supporting the purchase and distribution of the vaccine in very low-income countries.
So where we are now is a price that'll vary according to the scale, but at high volume it should be US$5 a dose.
Why has developing a malaria vaccine been so difficult?
People have been trying to make malaria vaccines for over 100 years. Well over 100 vaccines have gone into clinical trials in people. Very, very few have worked to any degree.
Malaria is not a virus, it's not a bacterium. It's a protozoan parasite, some thousands of times larger than a typical virus. A good measure of that is how many genes it has. Covid has 13, malaria has about 5,500. This is one of the reasons that malaria is super complex.
There are different parasite forms the first of which are injected by the mosquito into the skin and rapidly go to the liver. They spend a week multiplying there, and then they go into the bloodstream. And they are hugely different during these different stages. And the parasites grow at a rate of tenfold every 48 hours, multiplying furiously.
By the time they get to a really high parasite density, you will be very unwell. Or if you're unlucky, you will die, typically from cerebral symptoms, a coma or from being severely anaemic. The parasites break open the red blood cells.
And then there's yet another stage where the parasite changes again to a form that the mosquito can take up through its next bite and continue the life cycle by infecting somebody else.
So this is as complex as it gets with infectious pathogens.
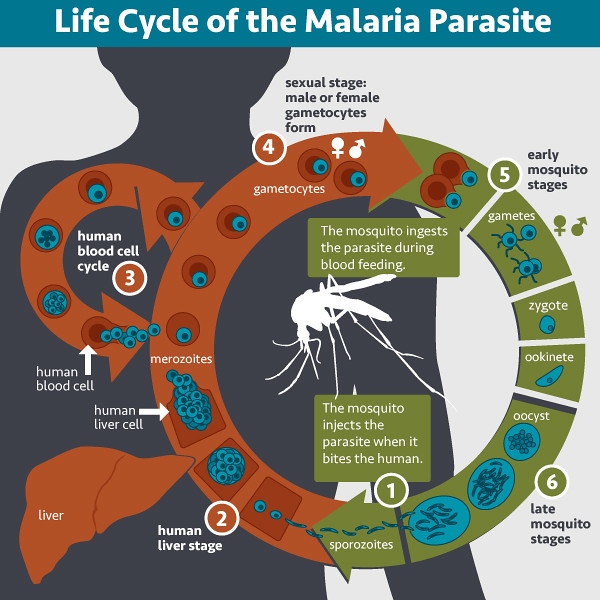
Malaria typically goes through four life cycles and they're all different. If you can get a really good vaccine for one of those, you will break the cycle of transmission. And that's what we've been trying to do.
We've been working on targeting the so-called sporozoites, which is the form that the mosquito inoculates into your skin. We're trying to trap it before it can get to the liver and carry on the life cycle.
Luckily, there are no symptoms of malaria at that stage. It's a silent infection until it gets into the blood and starts multiplying inside your red blood cells.
So the sporozoite is a natural target to try and kill the parasite before it multiplies very quickly.
Tell us about past attempts to develop a malaria vaccine
Very early on people tried to use the whole microbe in the same way that vaccine pioneer Edward Jenner used the whole virus to inoculate against smallpox. Then the French microbiologist Louis Pasteur came along with bacterial vaccines, and so on.
In about 1943, there was a trial of the whole malaria parasite vaccine candidate in New York with zero efficacy. That put people off for a while.
It wasn't until the 1980s when we could actually begin to sequence the genes in the parasite that new vaccination candidates appeared. And then within 10 years we had 5,000 candidates because everyone hoped that the gene they had sequenced might be a malaria vaccine. And of course almost all of those failed.
Why aren't vaccines for whole parasites effective against malaria?
It's the same reason that just getting infected once by malaria doesn't give you protection against the next infection.
In the areas of malaria where we test our vaccines in Africa, some children get up to eight episodes in three or four months. They get quite unwell with the first and three weeks later they're having a second bout and so on. Natural immunity doesn't work until you've had a lot of different infections and that's why adults are generally protected against malaria and don't become very unwell.
The people who die of malaria in an endemic area are the young children who may never have been infected before and die with their first infection when they're one year old, or they might have had one or two episodes, but that wasn't enough to give them sterilizing immunity.
Malaria has been around for tens of millions of years. Not just in humans, but in the species that we were before we became humans.
It's a very wily parasite and has developed immune escape mechanisms of all sorts.
When you try to vaccinate, you suddenly find there's some way the parasite gets around that, and it's only when you get up to really extraordinarily high levels of antibodies that the parasite hasn't seen before and hasn't learnt to evolve against that it becomes effective.
Will we ever eradicate malaria entirely?
Malaria is very high on the list of diseases we want to eradicate. I don't think it's going to happen in five years or 10 years, but it should happen in something like 15 years. So 2040 would be a reasonable target.
Nobody's suggesting we stop doing what we're doing at the moment with bed nets and spraying and drugs. But now we have a new tool that may be individually more protective than any of the tools we're using at the moment.![]()
Adrian Hill, Director of the Jenner Institute, University of Oxford
This article is republished from The Conversation under a Creative Commons license. Read the original article.
