Scraps of DNA discarded by our neurons' power units are being absorbed into our nuclear genome far more frequently than assumed, potentially putting our brains at greater risk of developing life-threatening conditions.
An investigation by a team of researchers led by Columbia University in the US has found individuals with higher numbers of nuclear mitochondrial insertions – or NUMTs (pronounced new-mites) – in their brain cells are more likely to die earlier than those with fewer DNA transfers.
Mitochondria serve as our cells' batteries, churning out energy in a form of chemical currency that suits most of our body's metabolic needs. Once a discrete microbial organism in its own right, these tiny powerhouses were co-opted by our unicellular ancestors billions of years in the past, genes and all.
Ever since then our ancestral and mitochondrial genomes have existed in a mutually beneficial share-house arrangement, with mitochondria slowly shuffling pages of their genome into the house's genetic library.
It's a sneaky migration that continues to this very day.
"It's rare, but a new NUMT becomes integrated into the human genome about once in every 4,000 births," says Columbia University molecular biologist Kalpita Karan.
"This is one of many ways, conserved from yeast to humans, by which mitochondria talk to nuclear genes."
For genealogists tracking mitochondrial inheritance or forensic clinicians building familial maps, such cross-contamination of genetic material can be a source of confusion.
Medical researchers have uncovered a more concerning problem: The insertion of mitochondrial DNA into our genomes can also interrupt vital processes.
"The mitochondrial DNA behaves similar to a virus in that it makes use of cuts in the genome and pastes itself in, or like jumping genes known as retrotransposons that move around the human genome," says University of Michigan geneticist Ryan Mills, who co-led the study with Martin Picard, a mitochondrial psychobiologist from Columbia University.
This 'home-grown' virus-like behavior has previously been associated with a heightened risk of specific cancers in other body systems, which together with evidence of NUMTs accelerating aging in yeast cells prompted researchers to consider whether a lifetime of accumulated mitochondrial DNA in non-reproductive nuclei might cause other health concerns.
Using a bank of post-mortem material collected as part of a separate longitudinal study on neurological problems in older people, the team led by Picard and Mills analyzed several different areas of brain tissue and immune cells represented across just under 1,200 individuals.
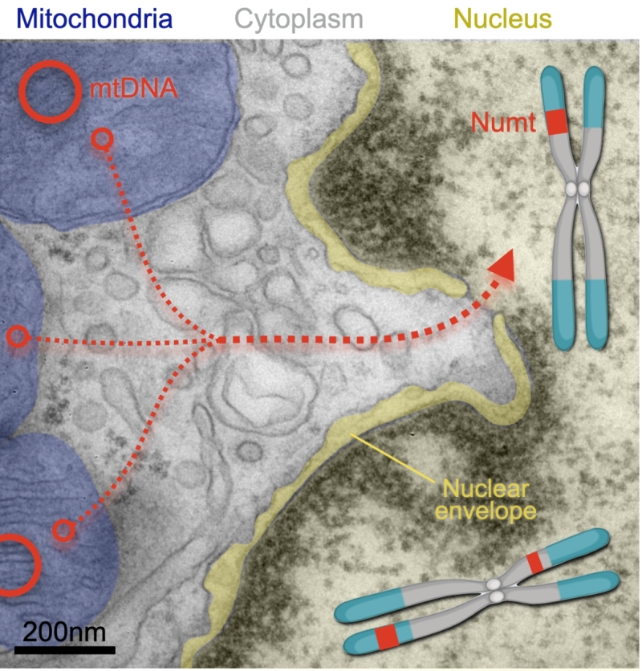
Not only were fragments of mitochondrial DNA being incorporated into the chromosomes of non-reproductive cells in the body surprisingly often, some particularly long-lived tissues harbored a lifetime of mitochondrial memories.
Compared with cells circulating in the blood, brain cells had accumulated far more NUMTs, with samples from the dorsolateral prefrontal cortex containing more than five times the number of mitochondrial insertions than tissues taken from the cerebellum.
Concerningly, individuals with higher numbers of NUMTs in their neural genomes were more likely to have earlier mortalities, with those who died younger presenting an additional two insertions for every decade of their life than their longer-lived peers.
"This suggests for the first time that NUMTs may have functional consequences and possibly influence lifespan," says Picard.
"NUMT accumulation can be added to the list of genome instability mechanisms that may contribute to aging, functional decline, and lifespan."

A measure of insertions in cultures of human skin fibroblasts found a novel NUMT appeared on average every 12.6 days, a rate that increased significantly among mitochondria containing stressful mutations.
It's not the first time discarded fragments of mitochondrial DNA have been suspected of causing neurological damage, yet it's now apparent their incorporation into our genetic library could be causing its own kind of chaos.
Analyzing other tissues across a wider population could reveal further insights into the consequences of mitochondria littering our cells with pages from their genetic diary.
Yet even this small glimpse into the genetic conversation inside our cells suggests we need to pay close attention to the age-old tension between these two ancient housemates.
This research was published in PLOS Biology.