Brains aren't the easiest of organs to study, what with their delicate wiring and subtle whispering of neurotransmitter messages. Now, this research could be made a little easier, as we've learned we can swap some critical chemical systems with the host animal being none the wiser.
In a proof-of-concept study run by a team of US researchers, the microscopic worm Caenorhabditis elegans was genetically gifted pieces of a nervous system taken from a radically different creature – a curious freshwater organism known as Hydra.
The swap wasn't unlike teaching a specific brain circuit a foreign language, and finding it performs its job just as well as before.
"There's a lot of diversity of synaptic connection in any animal's brain," explains Josh Hawk, a neuroscientist with the Marine Biological Laboratory in Massachusetts.
"Being able to pick and choose what to put in another organism will help us untangle and understand how and why brains do what they do."
Similar to us, the nematode C. elegans has a tightly-linked nervous system governed by chemical messengers called neurotransmitters. Different circuits make use of their own kinds of neurotransmitter, which are released into the thin gaps between neurons called synapses.
For the most part, these narrow voids are where a brain does much of its work. Synapses are the logic gates of the brain's computer circuitry – blocking some signals, enhancing others, transforming chemical fluctuations into something profound.
Neuroscientists can understand a great deal about the functions of a nervous system by tinkering with this traffic-light system using a variety of drugs, genetic tweaks, and light-operated switches.
Turning things on and off and watching the chaos can tell you a lot about how a nervous system operates. After all, much of what we've learned in neuroscience has emerged from observing the consequences of a broken brain.
"But to really understand how they work, you want to know if you can rebuild them – fix them – after they are broken. And that is very hard to do," says the study's senior author, Daniel Colón-Ramos from Yale University School of Medicine.
The trick in this case was to 'fix' a broken circuit in nematodes with parts borrowed from another organism, one that runs on very different biochemical software. Hydra aren't worms. They're more closely related to sea anemone, with tiny, tentacled bodies governed by a loosely-connected spread of neurons arranged in a simple, net-like structure.
Weirder still, the cells making up this neural mesh communicate with one another by squirting out peptides that then diffuse through the hydra's body, activating matching receptors on other cells.
"There are hundreds of neural peptides in Hydra, each of which could be a different channel of communication," says Hawk.
"To me, that's the most exciting thing. This should open up a whole area that no one has ever explored before."
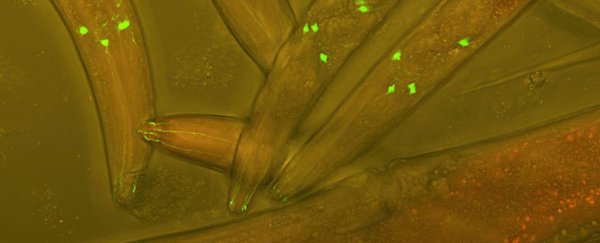
To test the concept, Hawk and his colleagues genetically altered specimens of C. elegans to lose their ability to feel full. These hungry worms exhibited foraging behaviors no matter how much food they'd consumed, giving the researchers a clear activity to watch for in their mutants.
From this group of worms they created two new lines – one with the gene for a hydra neuropeptide, and another with the corresponding receptor gene.
The offspring between the two families brought the two halves together into a single nervous system. Without their usual 'I'm full' brain circuit in operation, they had to rely on the hydra neuropeptides to signal an end to meal time.
The successful swap is just the first step. Thanks to the way hydra neuropeptides operate, it's possible to separate the neurons that use them to signal and have them communicate long-distance.
"It gives you more flexibility as a researcher to manipulate neurons that are not adjacent to each other," says Colón-Ramos.
This specific combination of messenger and receptor, dubbed HySyn, could be just the start of a vast tool-kit of replacement transmitters that researchers could use to decipher the intricacies of neural circuitry.
This research was published in Nature Communications.