People with Alzheimer's disease seem to be less likely to develop certain types of cancer, and a new study in rodents hints at why that is.
Among mice with symptoms of Alzheimer's, researchers in China noticed a lower incidence of colorectal cancer than is typical.
When these mice were given a stool transplant from a healthy mouse, however, their rate of cancer in the colon and rectum returned to normal.
The findings suggest that symptoms of Alzheimer's are closely linked to the makeup of the gut.
Today, evidence strongly indicates that certain intestinal microbes can shape the immune system in ways that impact the brain.
Several previous studies on rodents have linked the gut microbiome to symptoms of Alzheimer's disease. In recent groundbreaking experiments, stool transplants were even found to pass on memory impairment issues from one rodent to another.
The new research further investigates the close association between Alzheimer's, the gut microbiome, and cancer.
Some recent retrospective studies have found the risk of cancer in human patients with Alzheimer's is cut in half.
Meanwhile, the risk of Alzheimer's developing in patients with cancer is reduced by 35 percent. But no one really knows why that is, and colorectal cancer shows the strongest associations with Alzheimer's.
In a series of experiments at the First Hospital of Hebei Medical University in China, researchers found that mice with Alzheimer's-like symptoms showed resistance to colon cancer when the disease was artificially induced.
The intestinal inflammation among these mice appeared to be suppressed. When a fecal transplant from a healthy, younger mouse was given to the mouse with Alzheimer's-like symptoms, this suppression was lifted.
To figure out what microbes in the gut are pulling the strings, researchers sampled the microbiota of their animal models and came across several candidates, including a gram-negative bacteria, called Prevotella.
When mice were treated with Prevotella bacteria, the gut produced fewer pro-inflammatory immune cells, even when the mouse was exposed to dangerous pathogens.
The reduced inflammatory response probably occurred, in part, because the gut was 'leakier' than is typical, researchers explain, allowing certain microbial byproducts to enter circulation more easily.
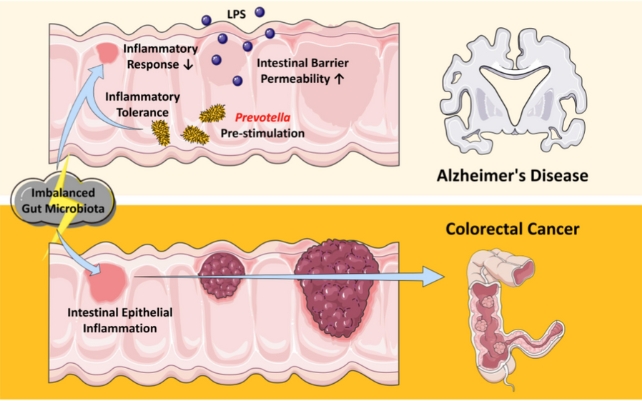
When mice were treated with Prevotella-derived compounds, the animals showed cognitive dysfunction and resistance to tumor development in their rectum and colon.
Many studies have shown that lipopolysaccharides (LPS) derived from the Prevotella genus are involved in mucosal barrier inflammatory responses, explain the researchers.
Prevotella bivia, for example, produces high LPS concentrations. These may create a toxic environment that damages dopamine neurons, which play a role in cognitive function and motor function.
A recent human clinical trial found evidence that stool transplants, for instance, can alleviate motor symptoms of Parkinson's, a disease that is closely linked to the degeneration of dopamine neurons.
Since inflammation is a major component of the process of tumor formation, it's possible that the anticancer properties in mouse models of Alzheimer's "may stem from the intestinal inflammatory tolerance induced by several specific bacterial genera in the gut microbiota," the authors of the study write.
Epidemiological data trends have previously shown patterns between cancer and Alzheimer's, but the new research now demonstrates a clear mechanism.
"This is biological/experimental evidence supporting an inverse relationship between the incidence of Alzheimer's disease and colorectal cancer," the team concludes.
The study was published in PNAS.