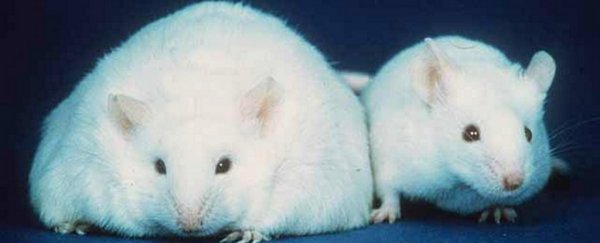
A drug that stimulates muscles to burn fuel as if they're exercising has been found to reduce fat mass in obese mice.
Developed by researchers in the US, the compound belongs to a proposed class of therapeutics known as exercise mimetics, that has been mostly tested in animals and is yet to be approved.
As the name suggests, these compounds-in-testing are designed to provide the benefits of exercise without the effort, switching on biological pathways that are typically activated during physical activity. Sounds good, right?
Promising though it seems, a lot more testing is needed before its true risks and benefits become clear. The human body's response to exercise is incredibly complex, after all. But this new drug candidate activates receptors found on high-energy demand tissues, such as muscles, producing effects in mice similar to repeated bouts of aerobic exercise.
So rather than suppressing appetite the way Ozempic does, animals treated with the drug burn more energy while resting and can exercise longer when they do start moving.
"This compound is basically telling skeletal muscle to make the same changes you see during endurance training," says Thomas Burris, a pharmacology researcher at the University of Florida and senior author of the study.
Our muscles mostly burn glucose during short bursts of exercise and weight training, switching to fatty acids during longer sessions of aerobic exercise such as running.
"When you treat mice with the drug, you can see that their whole-body metabolism turns to using fatty acids, which is very similar to what people use when they are fasting or exercising," Burris explains.
In a previous study, published earlier this year, Burris and his team showed their compound, SLU-PP-332, increased a specific type of muscle fibre in mice and enhanced exercise endurance. The normal-weight mice ran further and longer than untreated mice, as if the drug had unlocked new energy stores.
In this new study, the team wanted to understand more about the compound's effects on muscle metabolism, including the fuels used by the animals' muscles and changes in weight, glucose tolerance, and fat stores.
The researchers noticed that within two hours of administering the compound to mice, it prompted the animals' muscles to switch over to burning more fats like they would with prolonged exercise.
A month later, the effects of that switch became apparent. Regular mice treated with the drug for one month weighed much the same after treatment as they did before. The animals ate the same amount of food, and weren't any more active than usual, and yet their fat mass decreased when treated with SLU-PP-332.
In subsequent experiments with obese mice, the animals had similarly reduced fat mass after 28 days of treatment but also an average weight loss of about 12 percent. They simply expended more energy while resting.
"Our data clearly suggest that such compounds may hold utility in the treatment of diseases such as type 2 diabetes and obesity where exercise is typically prescribed," the researchers conclude.
However, the team's results on glucose tolerance and insulin sensitivity – two measures that relate to metabolic diseases such as diabetes – were a little bit mixed, so more research is needed to get a clearer picture of what's going on there.
The animals' lean muscle mass also didn't change significantly, though the researchers hope that their drug candidate might help prevent muscle wastage in older age.
Let's not forget either that physical activity has more perks than any medication can provide. It lifts mood, strengthens bones, improves sleep, and reduces stress – all benefits that can't be measured on bathroom scales.
The study has been published in the Journal of Pharmacology and Experimental Therapeutics.