A team of researchers from UNSW Science in Australia has created the most efficient water-slitting electrode to date, and it could be scaled up to allow industrial production of clean hydrogen fuel.
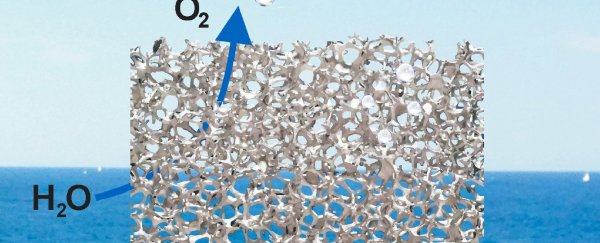
Hydrogen fuel is created by splitting water into hydrogen and oxygen using an electrical current. And although it has huge potential as a renewable energy source, right now it takes so much electricity to make it that it's not a feasible option. But this new electrode is made from a cheap, coated foam material that lets oxygen bubbles escape extremely quickly, making the whole process more efficient.
"Our electrode is the most efficient oxygen-producing electrode in alkaline electrolytes reported to date, to the best of our knowledge," said co-leader of the research, Chuan Zhao, from the UNSW School of Chemistry, in a press release.
"It is inexpensive, sturdy and simple to make, and can potentially be scaled up for industrial application of water splitting."
Unlike traditional water electrolysers, which use precious metals as the catalyst for the water-splitting reaction, the new technology uses cheap and widely available nickel and iron.
The device has now been described in Nature Communications, and is made from a nickel foam, which has holes in it about 200 micrometers across, or twice the diameter of a human hair. The nickel-iron catalyst also contains thousands of tiny pores, just 50 nanometers across, which makes it highly active and reduces the amount of electricity needed to split the water.
"The three-dimensional architecture of the electrode means it has an enormous surface area on which the oxygen evolution reaction can occur," said Zhao in the release.
"The larger bubbles of oxygen can escape easily through the big holes in the foam. As well, the smaller holes make the electrode surface 'wetter', so the bubbles do not stick to it, which is a common problem that makes electrodes less efficient."
The next step is for the scientists to further investigate the science behind the reaction and find ways to further boost the device's performance.
Importantly, if their electrode can make the process of splitting water less electricity-intensive, it will mean that we'll finally be able to unleash the potential of hydrogen fuel. Already hydrogen has great potential for powering mobile devices and vehicles and storing electricity generated from renewable energy sources, such as solar. And once we can create it in larger amounts, there'll be no stopping it.
"Cleaner sources of fuel like hydrogen will be particularly important for reducing carbon dioxide emissions and solving the air pollution problems from the burning of fossil fuels such as coal," said Zhao.
Love science? Find out more about the ground-breaking research happening at UNSW Science.