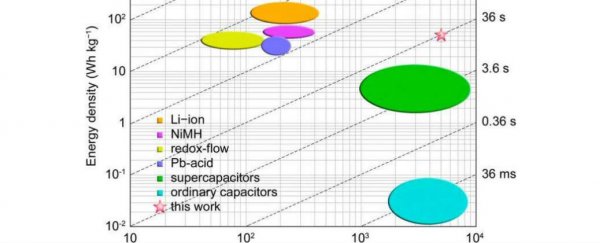
A new type of battery has been developed, and it displays qualities that are comparable to those of the lithium ion battery - one of the most commonly used power sources in consumer electronics - and the supercapacitor - a relatively new type of battery, valued for its capacity to release energy in large bursts.
Made from a combination of liquid ferrocene electrolyte, a liquid cathode, and a solid lithium anode, the new device has been called a 'semiliquid battery'. Based on what the team from the University of Texas at Austin in the US refers to as "a new chemistry", the device has been shown to keep up optical performance even after it's been charged and discharged at very high rates.
"The battery shows excellent rate capability that can be fully charged or discharged almost within one minute while maintaining good energy efficiency and reasonable energy density, representing a promising prototype liquid redox battery with both high energy density and power density for energy storage," one of the team, chemist Guihua Yu, told Lisa Zyga at Phys.org.
Combining the best elements of lithium-ion batteries with supercapacitors is something researchers have been getting pretty excited about over the past few years, because the technology could be used to not only make things much more powerful, it allows them to be much smaller and lighter too, with a charging time of just a few minutes, rather than several hours. This would be especially handy in hybrid electric cars and renewable energy resources, for which the efficient storage of energy is crucial.
Publishing in the journal Nano Letters, the team reports that their new semiliquid battery maintains stable cyclability with capacity retention of 80 percent over 500 charge and discharge cycles. As Zyga explains at Phys.org, the secret to the battery's success is that unique liquid electrode:
"The ions can move through the liquid battery very rapidly compared to in a solid battery, and the redox reactions in which the electrons are transferred between electrodes also occur at very high rates in this particular battery. For comparison, the values used to measure these rates (the diffusion coefficient and the reaction constant) are orders of magnitude greater in the new battery than in most conventional flow batteries."
So when does this thing make it to market then? Lithium-based batteries might be one of the most popular types of rechargeable batteries for portable electronics today, but under conditions can actually be very dangerous. So much so, that airlines around the world are now refusing to transport them in large quantities. So the challenge for Yu and his team now is to ensure the long-term stability and safety of the lithium anode component of their new battery.
"More advanced lithium anode protection is required to fully suppress self-discharge," Yu told Phys.org. "We suppose that other metals like zinc and magnesium may also function as the anode for such a battery as long as the electrolyte compatibility is resolved. We also expect that other organometallic compounds with multi-valence-state metal centres (redox centres) may also function as the anode, which eventually would make the battery fully liquid."
It's not there yet, but we can't help getting excited about just how much new technology like this could change the world as we know it. Bring it on.