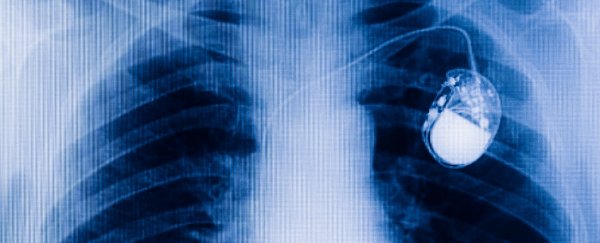
An international team of scientists has developed a tiny, wireless pacemaker that could one day save patients from having to undergo surgery. While traditional pacemakers are installed via a small incision in the chest, this new device is fed via a catheter through the leg and up to the heart, where it is attached to the right side of the organ.
Having been already implanted into 500 patients, the mini-pacemaker has caused significantly less complications than current pacemakers. But in those who did experience complications, the effects have been severe enough to hold back further implantations until the researchers can address these safety concerns.
"This is another landmark in the development of pacemakers," Christopher Granger of the American Heart Association, who was not part of the study, told the press, while adding that now is not the time to pursue the technology just yet. "I would tell patients to be careful of being one of the first to get this unless there's a compelling reason."
When a traditional pacemaker is installed, a 5-centimetre incision is made in the upper chest, and one or two wires are then fed into a vein in either the right or left side of the heart. These wires are connected to the pacemaker, which is then inserted beneath the skin near the heart.
By comparison, this new pacemaker - which is about 6 millimetres in diameter and 42 millimetres long - is fed through the body via a catheter that's inserted into the leg, and does not require wires to be installed in the heart. The researchers, who are based in Australia, the US, and Canada, say it will be appropriate for around 30 percent of patients in need of a pacemaker.
Statistically, the mini-pacemaker has so far caused less complications in the 500 patients who've had one for about six months - 7 percent of them reported side effects, whereas about 10 percent of regular pacemaker patients experience complications. This is because rather than have wires that are directly in contact with the heart, which can cause infections, block the vein, or break and become lodged inthe heart, all the wires in the mini-pacemaker are held inside its triple A battery-sized case.
According AP journalist Maria Cheng, the device has already been approved for use in Europe and is expected to be submitted to the US Food and Drug Administration (FDA) for approval shortly.
But there are still concerns about the technology in its current iteration. "A study of the device in Europe was twice stopped last year and in May when a worrying number of complications were reported, including one case where the device got dislodged and stuck in the artery leading to the patient's lungs," Cheng reports.
Plus the device is about twice as expensive as a regular pacemaker, and patients will need to see their doctor for regular check-ups, because it can't send information about their heart activity remotely. But despite all this, the researchers behind the device predict that people are still going to want it. "Patients are going to want this new pacemaker," lead researcher Vivek Reddy from Mount Sinai Hospital in New York told the AP. "It's up to doctors to talk to their patients about this, but patients will want (the smaller pacemaker) once they know about it."
The new pacemaker has been described in the New England Journal of Medicine and the results of the trial will be presented at a meeting of the European Society of Cardiology in London. The team will hopefully be working on making it safer than it has been so far, and will continue to monitor those that have already been implanted for signs of further complications.