Obesity impairs the human brain's ability to detect fullness and feel satisfied after sugar and fat consumption. What's more, the changes may be permanent, explaining why dieting can be a vicious cycle of weight loss and gain.
Researchers in the Netherlands and the US found that adults with medical obesity had different neurological responses to stomach infusions of dietary fat or sugar than lean adults.
Scans revealed a reduced release in dopamine, a neurotransmitter involved in creating feelings of 'reward' from food that help us recognize when we've had enough to eat.
Even after a significant amount of weight loss, volunteers were less able to register food in their stomachs, which may have profound effects on food intake.
The findings highlight a connection between the gut, brain, and obesity, showing it takes more than willpower to lose weight and keep it off.
"People still think obesity is caused by a lack of willpower," says Mireille Serlie, an endocrinologist at Yale University. "But we've shown that there is a real difference in the brain when it comes to nutrient sensing."
Research has changed the way scientists think about weight control in recent years, revealing it to be more complicated than personal control over calories and exercise, even when taking into account environmental factors like the pervasiveness of cheap, unhealthy junk food.
The complex biological mechanisms are being increasingly recognized and we now know people with obesity have to contend with a physiology that makes dietary changes and weight loss more challenging.
The researchers were interested in how fats and glucose individually trigger areas of the human brain connected to the rewarding aspects of food, without the confounding influences of taste and smell. In mice, the brain responds independently to nutrients in the gut, and while we are beginning to gain a better understanding of these processes in animal models, we know less about what happens in humans.
A part of the brain that regulates the drive to actively seek out and consume food piqued their interest in particular. Known as the striatum, it also plays a role in emotion and habit formation.
"We were specifically interested in this region as it has been proposed to function as a post-ingestive caloric sensor and to play an important role in the adaptation of feeding behavior to changes in the caloric value of energy intake," they write in their published paper.
To study this phenomenon in humans, Serlie and colleagues infused glucose or fat directly into the stomachs of 30 lean individuals with a BMI of 25 or less and 30 individuals with a BMI of 30 or higher. The people who took part were given infusions of either glucose, fat, or water (as a control) at random.
They used functional magnetic resonance imaging (fMRI) to analyze brain activity, and single-photon emission computed tomography (SPECT) to measure dopamine levels in the 30 minutes after the nutrients were given.
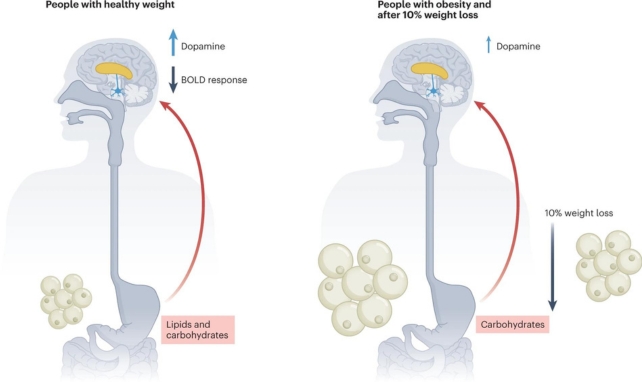
In lean participants, reduced brain activity in various regions followed infusions of glucose and of fat. After the nutrients were given, activity in the striatum went down and dopamine levels went up.
This overall drop in brain activity makes sense since we don't need to go get more food once we've already eaten. At the same time, rising dopamine levels showed that the brain's reward centers were working.
But the brain activity of the people in the obese category did not change. Their striatum remained active, and after the infusion of fats, dopamine, which is associated with the striatum's function, was not released in significant amounts.
Dopamine release was observed in response to glucose in both groups, although it didn't significantly affect brain activity in the people with obesity, and only the lean participants showed a response to fat.
"These impairments may contribute to overeating (and subsequent weight gain) and provide future targets for the development of therapies against obesity," the team writes.
Participants with obesity then underwent a 12-week dietary weight-loss program, and in the 26 who achieved at least a 10 percent weight loss, the tests were repeated as before, with surprising results.
"None of the diminished responses were recovered," Serlie explains.
The findings suggest that reduced nutrient sensing in the stomach and gut and impaired brain responses to nutritional signals may have profound effects on food intake, obesity, and the struggle with weight loss and regain.
Precisely when such profound changes occur during weight gain isn't clear. Given the small size of the test groups, some caution is required when it comes to interpreting the findings.
Nonetheless, the results provide important new details that further imply brain signaling is a result of obesity rather than a cause, laying vital groundwork for future investigations.
"We need to find where that point is when the brain starts to lose its capacity to regulate food intake and what determines that switch," says Serlie.
"Because if you know when and how it happens, you might be able to prevent it."
One important take home message here is that it's clear, once again, that weight stigma has no place in the battle against obesity.
The peer-reviewed study has been published in Nature Metabolism.