Given how profound an influence opioids have on the world – for better or worse – scientists know surprisingly little about how these drugs actually work.
A recent study led by researchers from University of California Davis describes a new method by which researchers can watch in real-time as the nervous system responds to the activation of its opioid receptors.
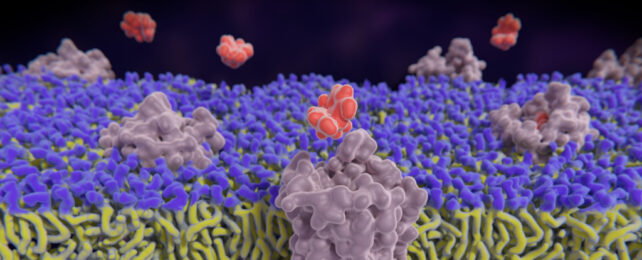
By incorporating fluorescing receptors in neurons of mice, the team showed it was possible to visualize the activity of opioids as they operated inside a living brain.
For many people, the word 'opioid' brings to mind drugs like heroin and fentanyl. However, the human body has its own opioid system, which is an integral part of the brain's reward and aversion pathways.
This system revolves around three receptors referred to as kappa (κOR), delta (δOR) and mu (µOR), which are activated by a variety of short-chained amino acids released by neurons in response to pleasure, pain, and stress. The best-known of these opioid neuropeptides are probably the endorphins – a portmanteau of 'endogenous morphine' – but there's a whole heap of them.
Opioid drugs also bind strongly to, and activate, our opioid receptors. While there are non-psychoactive opioid drugs – an example is the anti-diarrheal drug loperamide – most of these drugs suppress pain and induce strong feelings of euphoria, while forcing the brain to adapt to a new opioid baseline. This makes them valuable as painkillers, but also makes them highly addictive.
This much we know. However, there are all sorts of subtleties and intricacies to what seems at face value a fairly straightforward process. As the paper points out, even the process of understanding how neuropeptides interact with the opioid system is difficult: "[The receptors] can be activated by at least 20 endogenous opioid peptides with differential affinity and selectivity".
The point about "differential affinity and selectivity" is important, because different opioids bind more strongly or weakly to each receptor. This means that the degree to which each receptor is activated is more like a light bulb on a dimmer than it is a simple on/off switch.
The tiny quantities of neuropeptides involved in the opioid system have also proven a hurdle. The paper notes that "the released concentration may also be at orders of magnitude lower than classical neurotransmitters … As a result, it has been exceedingly difficult to study the processes that regulate opioid neuropeptide release."
And, of course, there are far more synthetic opioids than there are neuropeptides, all of which affect the brain in subtly different ways.
The paper describes a new technique for studying exactly what happens at our opioid receptors when they encounter something to which they bind, be it an endogenous neuropeptide or an opioid drug.
The technique involves three carefully tuned molecules called biosensors, one based on each of the κOR, δOR and µOR receptors.
These molecules fluoresce as a substance activates the receptor in question, and this fluorescence fades as the receptor returns to an inactive state. The fluorescence is dose-dependent, and is also diminished by opioid blockers like naloxone, which bind strongly to receptors without activating them.
Applied to mice with fluorescent biosensors in their brain's hippocampus region, the team was able to watch the opioid receptors respond to different drugs and neuropeptides.
The findings promise to not only advance our understanding of the opioid system, but benefit the search for potential treatments for anxiety and depression, not to mention the seemingly eternal quest for a drug that provides the pain relief of existing opioids without the accompanying potential for addiction.
The paper has been published in Nature Neuroscience.