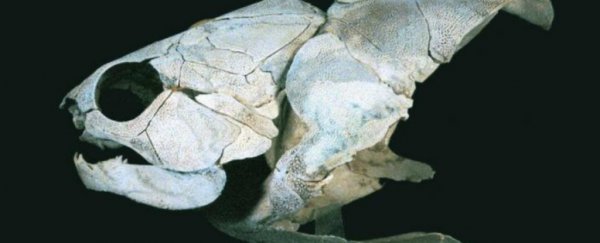
The placoderms were a diverse group of ancient armoured fishes and it's widely believed that they are ancestral to virtually all vertebrates alive today, including humans.
Placoderms dominated aquatic environments for 70 million years until they suddenly went extinct some 360 million years ago, paving the way for modern bony fish (osteichthyans) and sharks and rays (chondrichthyans).
The earliest vertebrates were jawless fishes, and placoderms were among the earliest fishes to evolve jaws, an adaptive breakthrough that contributed to their rapid success.
Several studies have strongly argued that placoderms are the direct ancestors of all other jawed vertebrates, a huge branch of the tree of life that includes mammals, birds, reptiles, amphibians, and most fish.
But our new research, published today in Systematic Biology, raises the possibility that placoderms could be just a bizarre evolutionary dead end.
Are we all armoured fish?
If all jawed vertebrates, including humans, are nothing more than highly evolved placoderms, then key features of ourselves should be traceable to structures that first appeared in our fishy placoderm ancestors. This would include particular jaw and skull bones and the proportions of our face and brain.
But our new evolutionary tree challenges the idea that placoderms gave rise to all other jawed vertebrates.
Instead, we suggest they are a side branch in vertebrate evolution – diverse and successful in their day but ultimately all destined for extinction. If correct, this alternative tree would require a radical rethink of many aspects of vertebrate evolution.
 Benedict King/Brian Choo/Flinders University
Benedict King/Brian Choo/Flinders University
Evolutionary trees (depicting genealogical relationships between species) are of great interest to scientists because they reveal a lot about the process of evolution. For example, they can tell us how humans evolved from great apes, or how HIV spread around the world.
Making such trees for extinct animals is notoriously difficult. DNA is only retrievable from the most recent fossils, so palaeontologists typically rely on skeletal features preserved in fossils to infer these relationships. Basically, species with lots of features in common are likely to be close kin.
Such studies are often confounded by the fragmentary nature of the fossil record.
Another problem occurs when trying to work out relationships between placoderms, jawless fish and other early vertebrates. Many of these groups are so utterly different from each other that anatomical comparisons are difficult.
Imagine trying to identify and compare equivalent bits of anatomy shared between an oyster, a beetle, and a blue whale. That is essentially the problem we face with early vertebrate fossils.
A new approach to inferring genealogies
This is where our new methods come in. We used a sophisticated new model for producing genealogies that not only looks at anatomical features, but also considers other sources of information, such as the geological ages of the fossils and how much evolution they have undergone.
Very old, primitive fossils are likely to sit on low branches of the tree, whereas young, highly evolved fossils are likely to sit on twigs near the crown.
We theorised that this method might be better than looking at anatomy alone, due to the difficulties in comparing jawless and jawed vertebrates.
When we ran the analysis, it produced a completely different result from other recent studies. Placoderms, instead of being the primitive stock from which all other jawed vertebrates were descended, were instead a distinct side branch which left no living heirs.
 John Long/Flinders University
John Long/Flinders University
This new tree forces a rethink of some major events in vertebrate evolution. For example, placoderms copulated, possessing bizarre bony external genitalia for internal fertilisation, whereas other early jawed vertebrates appear to have spawned like salmon do.
If placoderms were ancestral to other jawed vertebrates, then placoderm-style reproduction must have come first, subsequently giving way to salmon-style spawning.
But if placoderms are a specialised side branch, the scenario reverses. Spawning was primitive and the unusual reproductive biology of placoderms becomes one of the specialisations of this evolutionary dead end.
To most biologists, the latter intepretation makes a lot more sense.
Our tree also suggests a more complex scenario for the evolution of the modern vertebrate face. In addition, the jaw bones of placoderms can no longer be assumed to be primitive. In fact, our own jawbones may be, in some ways, more primitive than the specialised jaws of a placoderm.
So what did our fishy ancestors look like?
So if placoderms were not our ancestors, what was? Our study suggests that no particular group of known jawed vertebrates is ancestral to the others.
Rather, the true jawed vertebrate ancestor probably combined features of osteichthyans (bony fish), chondrichthyans (sharks and rays) and placoderms in much the same way that the common ancestor of humans and chimpanzees was neither human nor chimp, but a unique precursor of both.
The new models we employed also revealed that jawed vertebrates probably underwent a period of rapid evolution even before they first appear in the fossil record, around 424 million years ago.
Fossil discoveries from this key unknown period are required to unravel the mysteries of the origin of jawed vertebrates.
Fantastic progress is already being made with new fossil discoveries from China, which are among the oldest jawed vertebrates known. These fossils combine placoderm and osteichthyan features, and may be our greatest clue yet of what the ancestors of jawed vertebrates looked like.
 Benedict King/Brian Choo/Flinders University
Benedict King/Brian Choo/Flinders University
Our paper is likely to divide opinion, as the methods used are still in their infancy and yet to be widely adopted by palaeontologists. The debate will doubtless continue into the future.
But what everyone agrees on is that study of early vertebrate fossils such as placoderms is vital for unravelling the evolution of the wonderful diversity of fish, amphibians, reptiles, birds and mammals that today populate our planet.
Benedict King, PhD candidate in vertebrate palaeontology, Flinders University;
John Long, Strategic Professor in Palaeontology, Flinders University,
Mike Lee, Professor in Evolutionary Biology (jointly appointed with South Australian Museum), Flinders University.
This article was originally published by The Conversation. Read the original.
![]()