The idea of freezing particles by warming them is counterintuitive, to say the least. But physicists have shown how specially designed mixtures 'melt' in the dark but crystallise the moment the lights come on, thanks to their unique thermal activity.
Instead of bouncing the particles around and spreading them out, the researchers showed that by using light to heat up the mixture, they were able to lock particles in place and force them to clump together, as if they were frozen.
Researchers from the University of Cambridge in the UK carried out their experiments on a colloid made up of water, polystyrene and small droplets of oil coated in DNA to better understand the dynamics taking place between them when warmed by light.
As your high school physics teacher once drilled into you, particles suspended in a temperature gradient flow away from hot spots towards cooler ones.
So it'd stand to reason that if we heated suspensions of oil, focussing on the boundary with its watery surrounds, you would expect the mix of molecules to jiggle with excitement, bumping and grinding their way towards cooler areas and causing the fluids to move.
There's even a term for this oil and water flow; the Marangoni effect.
Putting it simply, the contrasting surface tension between oil and water makes each susceptible to variations in temperature in slightly different ways, forcing their particles to scatter.
To study the effect light has on suspensions of droplets, soft matter physicist Alessio Caciagli and his team coated 20 to 30 micrometre-wide blobs of oil in a polymer that was heavily dusted with single strands of DNA.
These fuzzy oil balls were then combined in a suspension with polystyrene spheres roughly half a micrometre in diameter. The DNA connected the polystyrene to the outer surface of the oil drops, so when the material was suspended in water it formed a loosely bound colloid.
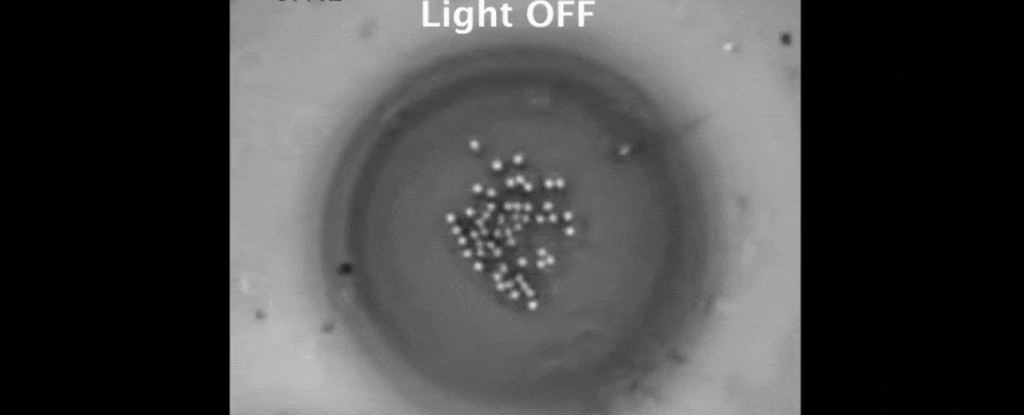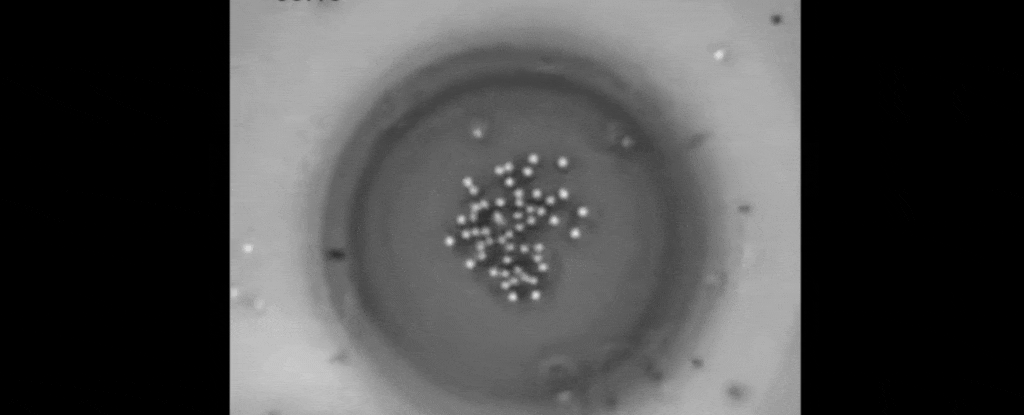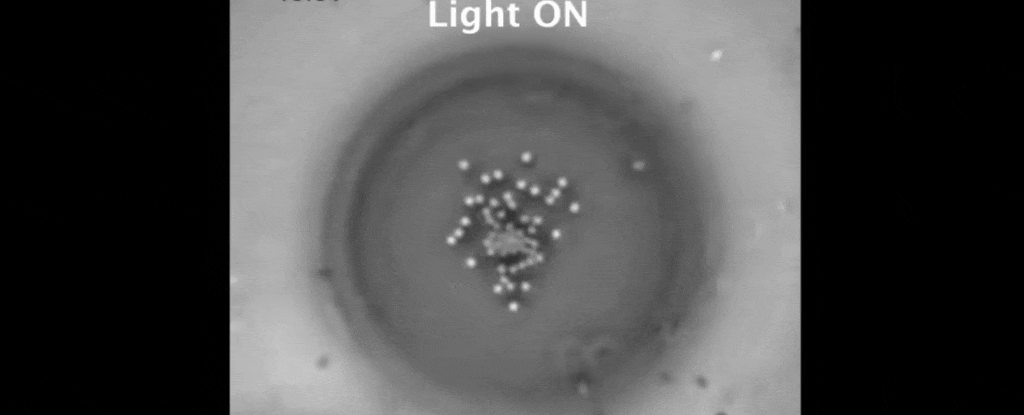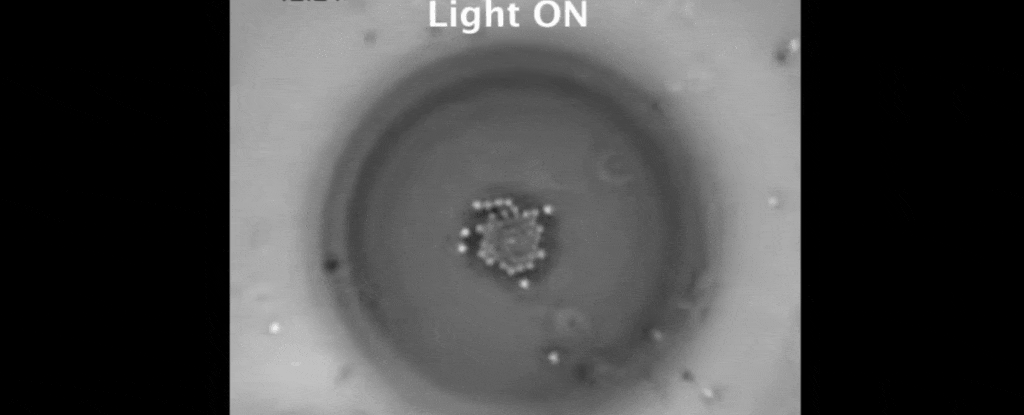
Then the real fun began. Shining a light on the interface between the oil and the water caused a single polystyrene clump to sit in place, gripped by well understood optical effects.
Basking in the laser's glow, the polystyrene's temperature rose by about 5 degrees Celsius, setting up a heat gradient against the surrounding water.
Ordinarily the Marangoni effect should be enough to scatter the polystyrene spheres and send the colloid flying apart.
But tethered together by a hazy mesh of DNA strands, the polystyrene instead drifted closer together on the surface of the oil droplet.
It turns out, the heat gradient produced by the trapped polystyrene creates flow in the two liquids that suck the other suspended particles in close.
The result is a peculiar crystallisation of colloidal particles triggered by the warmth of a light beam. To make them melt, all that's needed is darkness.
Take a look for yourself in the clip below.
Although it's not what we might have expected, the physics all seems fairly fundamental, and was tested by the team's models.
Light is proving to be a rather versatile instrument for manipulating particles. As the endless miniaturisation of technology demands ever-more-precise tools for pulling and poking super tiny materials, we're inspired to invent nanoscale equivalents of forceps and hammers based on the complex effects light has on matter.
Using the optical properties of a laser to create a 'heat magnet' for suspended materials is just one more way we might one day pull together the molecular scale machines of tomorrow.
This research was published in Physical Review Letters.