Nearly a century after experiments confirmed that atoms, matter's smallest building blocks, have ethereal, wave-like characteristics, physicists have just found a new way to show how mammoth-sized molecules ripple with the same uncertainty.
Researchers from the University of Vienna and the University of Duisburg-Essen, Germany, put a new spin on a classic experiment to create wave-like diffraction patterns in two kinds of organic chemicals.
This is a big deal not only because it once again demonstrates the strange duality of the particles that make up our world, but it could help improve methods for important imaging materials.
Put simply, the researchers used a laser to create mists of individual molecules made up of around 40 to 60 atoms: in one case they used the antibiotic ciprofloxacin; and in another the organic dye phthalocyanine.
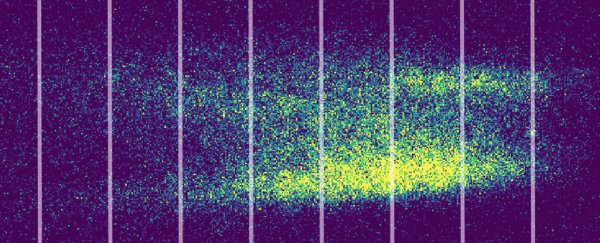
Each mist was passed through a series of narrow openings and then a second laser, before splashing onto a screen.
Illuminated with a UV light, the mist that passed through revealed the tell-tale pattern of waves interfering with themselves mid-flight.
But how can physical matter act like waves? When we think about things on a human scale of dogs and cats and apple pies and tennis balls, it's hard to explain how ordinary particles suddenly start acting like 'waves' of sound or light.
And it's not just us - coming up with comparisons has challenged the best minds in physics as well.
In the early days of atomics, it was accepted that light was like a ripple across the water's surface. This was clear because when a beam of light is blocked, its properties appear to project from around the edge of the obstacle. Or, in simpler terms, it can seem to warp and 'diffract' around corners, like waves bending around reeds emerging from a pond.
Importantly, waves can also build or subtract from one another when they overlap, interfering with their pattern in predictable ways. Light, it happens, does this as well.
Matter – such as the negative and positive charges making up nature's fundamental building blocks – was thought to be more like tiny grains of sand at the beach. Pile them together, and they just form a mound.
By the beginning of the 20th century, it was becoming clear there was more to the whole story.
Einstein would earn his Nobel stripes for experiments that revealed light not only behaves like a wave, it also delivers energy in discrete, grain-like units.
A couple of decades later, a young French prince named Louis de Broglie took a leaf from Einstein's book by suggesting if the granular electrons were also wave-like, it could explain their weird orbital nature around atoms.
The de Broglie's crazy idea wasn't just waffle, either. In 1927, an experiment by physicist George Thomson showed electrons can diffract through narrow openings to create interference patterns just like any other wave.
Ever since then, the evidence in support of this strange duality in light and matter has piled sky-high. We're not just talking rainbows and electrons here, either; the very foundations of physics are described using the mathematics of both waves and particles.
Those quivering electrons combine with wobbling protons and quaking neutrons, all shimmying through reality on waves of confusion, never quite sure of their destiny or their personality until they're forced into one.
As those particles connect together to form atoms, and atoms join to form molecules, and molecules combine into apple pies and tennis balls (and even humans like you and me), those waves coalesce into more obscure, less easily spotted forms.
But they're still there, if you know how to look. Just as this experiment shows.
As far as sheer size goes, this particular study is no record-breaker. Researchers revealed the wave-like nature of a juggernaut molecule made up of 810 atoms just over seven years ago.
In fact, phthalocyanine was captured in wave form back in 2017, using a slightly different setup to this one.
The difference this time was in how the team diffracted the waves, substituting into Thomson's famous original experiment a diffraction process based on Bragg's laws instead of the more traditional Raman-Nath diffraction.
To most of us, this subtle change won't mean a great detail. But researchers could use this new technique to create diagnostic tools that give us new ways of exploring a wider variety of particle properties.
"The possibility to selectively address the arms in such a setup would, in turn, enable new interference schemes utilising the molecules' chirality, conformation, and possibly entanglement between the molecules' internal and external degrees of freedom," the researchers conclude in their report.
Having insight into all of those quantum traits could give us insight into the ways atoms fit themselves together, helping us better predict processes for manufacturing new materials.
It might even tell us a few new things about the nature of waves and particles themselves, finally allowing us to reconcile the immiscible halves of reality once and for all.
This research was published in Physical Review Letters.