According to the basic laws of thermodynamics, if you take a warm apple pie out of the oven and place it on a window sill, it will eventually cool to be the same temperature as the surrounding air.
But physicists have discovered that under certain circumstances, charged particles called ions don't follow this logic - in fact, they end up cooling to two entirely different temperatures.
"This apparent departure from the familiar laws of thermodynamics is akin to our warm apple pie either cooling as expected, or spontaneously bursting into flames, depending on the pie's exact temperature when it is placed in the window," says one of the team, Eric Hudson, from the University of California, Los Angeles.
Before trying to observe the hidden quantum mechanical properties of particles, physicists will often cool them right down, because this slows their movements and allows for greater observation and control.
To cool down ions, they use a technique called buffer gas cooling, which effectively 'traps' ions and exposes them to clouds of cold atoms.
Every time ions collide with the atoms in the clouds, energy is transferred between the two, until eventually the ions and the atoms reach the same cool temperature, in theory, just like our hypothetical apple pie.
At least, that's what physicists have assumed. But Hudson and his team have, for the first time, shown that reality is far more complicated - and weird.
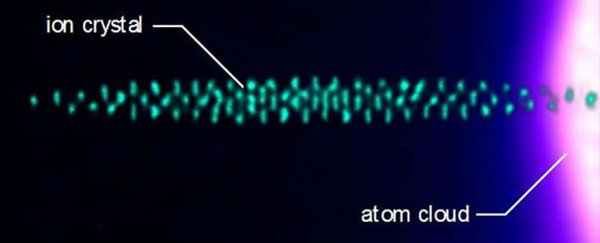
To test the behaviour of ions in an ion trap, the team prepared a sample of laser-cooled barium ions, and a sample of laser-cooled calcium atoms. Both had been cooled to a mere one-thousandth of a degree above absolute zero.
The ions were then immersed in clouds of around 3 million super-cooled calcium atoms, and held in place by electric fields that oscillate so fast - millions of times every second - that it forces the ions to levitate in a set position smaller than the width of a human hair.
The researchers allowed the super-cooled ions and atoms to mingle and collide with each other in this set-up for a while, and then measured their resulting temperatures.
Instead of finding that the two shared the same temperature, they recorded multiple final temperatures among the ions, which appeared to depend on the number of ions that were cooled at the same moment, and what their exact starting temperature was.
The results suggest that buffer gas cooling is a far more complex process than physicists have realised, and is not able to achieve the temperature equilibrium they were expecting.
When you've got everyone from forensic investigators to particle physicists who are trying to produce antimatter relying on the effectiveness of this technique, that inconsistency is something they need to account for.
"Our results demonstrate that you can't just throw any buffer gas into your device - no matter how cold it is - and expect it to work as an effective coolant," says one of the team, Steven Schowalter, from NASA's Jet Propulsion Laboratory.
The study has been published in Nature Communications.