Two clinical trials have confirmed suspicions – an innovative treatment for a rare neurological condition typically emerging in childhood also increases cancer risk.
Phase 2/3 studies on the effectiveness and safety of a US FDA-approved gene therapy for cerebral adrenoleukodystrophy have delivered bittersweet findings, verifying the treatment's success while showing it comes with a significant risk of a cancer-causing disorder – myelodysplastic syndrome (MDS).
Following treatment, six out of 67 patients across the two recent clinical trials developed MDS, with an additional patient developing leukemia. Further analysis of biopsied cells revealed a variety of genetic insertions that are likely to have contributed to the conditions.
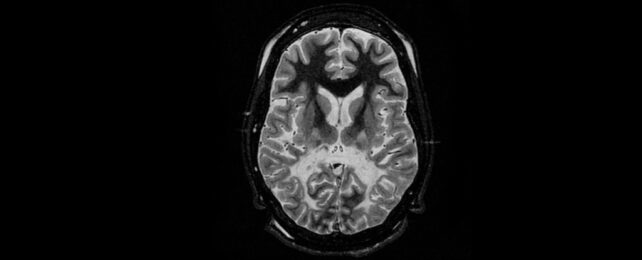
Cerebral adrenoleukodystrophy (CALD) results from a mutation in the ABCD1 gene on the X chromosome that compromises the body's ability to break up very long-chain fatty acids, causing them to accumulate in areas like the nervous system.
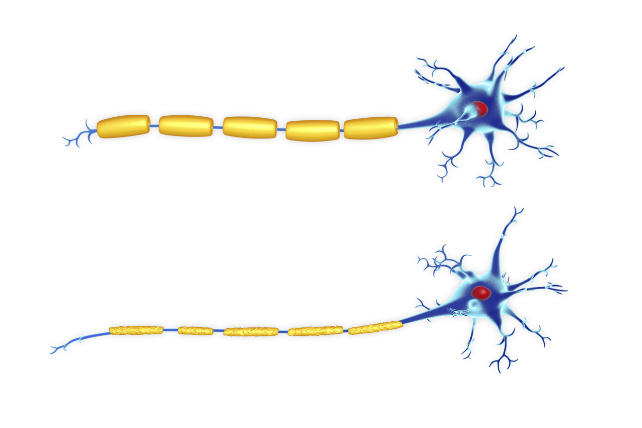
The consequences can be devastating, destroying the protective sheath surrounding the nerves' axons and gradually leading to a decline in cognitive abilities and motor functions. Left untreated, the condition can be fatal.
"Cerebral adrenoleukodystrophy is a devastating brain disease that strikes children in the prime of their childhood and development," says neurologist Florian Eichler, director of the Leukodystrophy Clinic at Massachusetts General Hospital.
Being an X-linked abnormality, men are affected far more than women, particularly earlier in life, with symptoms beginning to appear between ages 4 and 10 in just over a third of those with the mutation.
Should the disease be diagnosed at a sufficiently early point in the disease's progress, children can undergo a form of stem-cell therapy where genetically-matched blood cells from a donor are transplanted into the patient, providing them with a working ABCD1 gene.
Though highly successful, the treatment is effectively a tissue transplant, complete with an elevated chance of side-effects, immune responses, and potential for rejection.
What's more, not all patients are eligible for the treatment, which requires a compatible donor.
In 2009, a collaboration between French and German researchers demonstrated a modified HIV virus could be used to insert a working ABCD1 gene into blood stem cells removed from a patient's own body, removing the need for – and risks of – using donor tissue.
That has since been developed into a treatment called elivaldogene autotemcel. Sold as Skysona by the company Bluebird Bio, it received the thumbs-up from the FDA in 2022. Clinical trials found those receiving the genetic therapy had a 72 percent chance of being free of six major functional disabilities in the two years following their first CALD assessment, compared to 43 percent for controls.
Given claims that approximately one out of every two young patients don't survive more than eight years without treatment, every success feels worthy of celebration.
Yet it was already becoming clear the treatment wasn't risk-free. An analysis of continued clinical trials has now provided strong evidence of a one in ten chance of developing a cancerous disorder.
It's possible further patients could develop cancer over the longer term as a result of the therapy. Fortunately, leukemias are responsive to a range of treatments; six of the patients received further treatment, with four becoming cancer-free.
"When I initially began treating patients with CALD, 80 percent came into our clinic on death's door, and now the ratio has flipped," says Eichler.
"We cautiously celebrate that we have been able to stabilize this neurologic disease and give these boys back a fulfilling life, but that jubilation is dampened by the fact that we see malignancy in a subset of these patients. This is something that we are actively trying to understand and address."
Medicine is full of agonizing decisions that weigh chances of living a longer, healthier life against the risk of adverse outcomes. Only through clinical trials such as these can patients and their families have sufficient information to make tough choices.
As stated by Boston Children's Hospital oncologist David A. Williams, "While the risks associated with gene therapy and vector technology are real, the progress we've made offers a source of hope for families facing limited options."
This research was published in the New England Medical Journal here and here.