A new model of Alzheimer's disease has been proposed, which could speed up efforts to understand and cure the complex condition – while bringing all manifestations of the condition under one unifying theory.
Researchers from Arizona State University suggest that stress granules – protein and RNA clumps that form around cells in stressful conditions due to genetic and environmental risk factors – are the primary culprit behind the disease.
In their new study, the team reviewed data from multiple health databases and past papers – particularly a 2022 study on Alzheimer's progression – to identify widespread changes in gene expression that come with it.
We know Alzheimer's makes these wholesale changes early on, effectively rewiring biological pathways to increase cell stress, block neuron communications, and cause protein abnormalities, such as amyloid-beta clumps.
What's less clear is what's behind this disastrous shift in gene behavior – and whether this could also explain Alzheimer's.
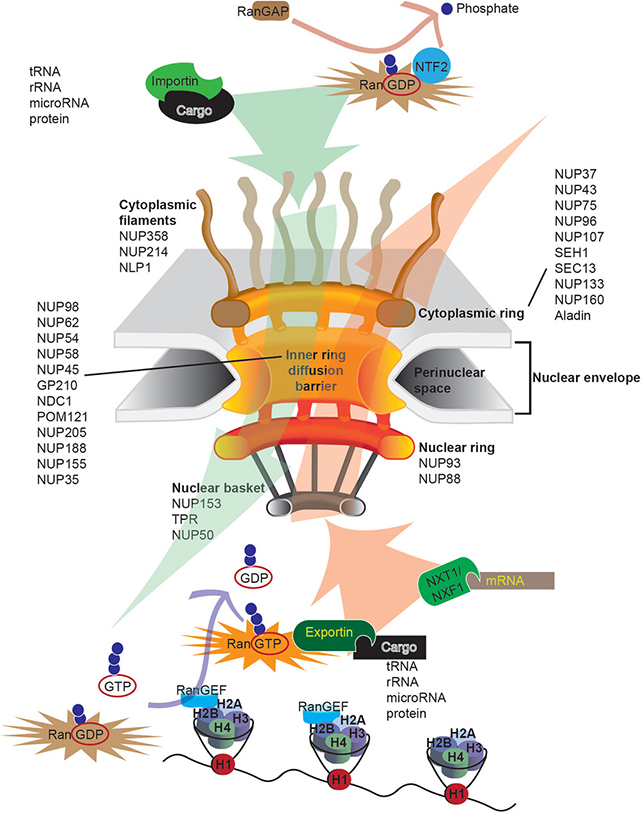
These stress granules are believed to protect cells while homeostasis is restored, but in Alzheimer's, the indications are that they persist and disrupt other processes – including, significantly, nucleocytoplasmic transport. That's where crucial molecules are moved between the nucleus of the cell and its surrounding cytoplasm.
"Our proposal, focusing on the breakdown of communication between the nucleus and cytoplasm leading to massive disruptions in gene expression, offers a plausible framework to comprehensively understand the mechanisms driving this complex disease," says neuroscientist Paul Coleman.
"Studying these early manifestations of Alzheimer's could pave the way for innovative approaches to diagnosis, treatment, and prevention, addressing the disease at its roots."
The hypothesis puts forward the idea that stress granules disrupt the cell transport system, which then modifies gene expression, and then that in turn causes all the symptoms of Alzheimer's – including neuroinflammation and tau protein tangles.
In other words, all the different facets of Alzheimer's disease could come from the same source. While there's no firm proof yet that this is what's happening, the researchers have demonstrated that it plausibly fits the current evidence.
And because this cell stress happens before any Alzheimer's symptoms, it gives scientists an opportunity to try and block the disease at its earliest stages. It's possible that most symptoms could be prevented at the source.
A variety of factors, from air pollution to genetic mutations, could be triggering these stress granules to linger longer – and future studies will be able to look in more detail at how they're formed and how they cause damage.
"Our paper contributes to the ongoing debate about when Alzheimer's truly begins – an evolving concept shaped by advances in technology and research," says Coleman.
"The key questions are when it can first be detected and when intervention should begin, both of which have profound implications for society and future medical approaches."
The research has been published in Alzheimer's and Dementia: The Journal of the Alzheimer's Association.