New findings by scientists in Australia could challenge what we thought we knew about the way gold nuggets bloom in vast reefs beneath our feet.
Under pressures of hundreds of megapascals (tens of thousands of pounds per square inch) and boiling hot temperatures, water squeezed up from the depths of Earth's crust carries dissolved gases, metals, and minerals to the surface with every quake and shudder of a seismic event.
As any good prospector knows, buried seams of crystalized silicon dioxide – better known as quartz – are fertile ground for gold mining, with both materials precipitating out of solution under strikingly similar conditions.
Though the basic mechanisms behind the precious ore's formation have been understood for some time, certain details have never quite added up, and new research from scientists at Australia's Monash University, the CSIRO, and the Australian Nuclear Science and Technology Organisation challenge the conventional views on how gold forms.
"While this theory is widely accepted, it doesn't fully explain the formation of large gold nuggets, especially considering that the concentration of gold in these fluids is extremely low," says Chris Voisey, a geologist at Monash University.
As an element, gold doesn't dissolve easily in water, making it rare to find concentrations above one part per million. On the other hand, deposits of gold represent an incredible degree of enrichment, many thousands of times more concentrated than the diffuse solution that creates them.
A number of geological and biological processes could account for substantial lumps of gold ore accumulating in some locations. Dustings of gold could also fall out of solution before concentrating in one spot.
Only none of this explains why gold particles might settle inside a block of quartz, collecting as chunks big enough to make a fevered digger yell "Eureka!". So Voisey and his team wondered if the pairing of gold and quartz might be more intimately connected than first seemed.
Silicon dioxide is an incredibly unique material. Where other crystals are relatively symmetrical, quartz forms with a bias that produces a voltage when stressed – a phenomenon known as the piezoelectric effect.
With every tremor of Earth's crust, seams of quartz will crackle with static currents as voltages emerge and electrons rebalance.
This charge jump is unlikely to move very far given quartz is an insulating material. Gold, on the other hand, is a great conductor of electricity, raising the possibility that electrochemical reactions within quartz seams might serve as a catalyst, drawing sufficient gold from solution in concentrated spots through repeated cycles of tiny shakes.
"Earthquake seismic wave frequencies vary wildly based on magnitude and rock composition, but range from 1 hertz to over 20 hertz," Voisey explained to ScienceAlert.
"Each of these waves can distort the quartz crystal and cause a piezoelectric voltage to build, which will have some chance to reduce gold from nearby solution."
To test whether this reduction was sufficient to cause grains of gold to grow in size, the researchers placed a dozen small quartz tiles cut from natural crystal into aqueous solutions of gold.
Half of the slabs were then jiggled 20 times a second for an hour to replicate a small quake, producing voltage of between 0.4 and 1.4 volts. The other half were left alone in their baths to act as controls.
Analysis with a scanning electron microscope revealed micrometer-sized gold grains had formed on the jiggled tiles, while none appeared on the controls.
Subsequent tests using quartz tiles naturally speckled with grains of gold revealed the tiny 'seeds' bloomed further in solutions when a stress was applied. Crucially, these tiny gold foundations took priority as nucleation sites of ore formation, with their presence reducing the chance of new gold grains forming nearby.
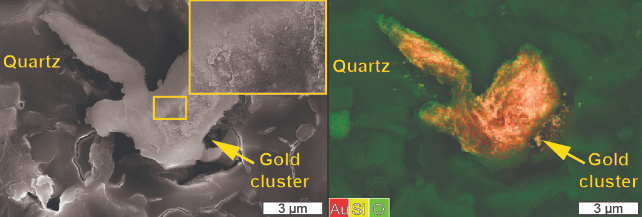
"Once some gold has been deposited, this chance increases because gold will behave like a catalyst for further reaction due to its conductivity," Voisey explained to ScienceAlert.
What was simulated in the lab using concentrated solutions and extensive periods of shaking would of course take far longer in the real world with dilute solutions and occasional tremors.
On geological timescales, however, the process could be relatively rapid. Without the added zap of stressed quartz, it's difficult to even explain how gold might accumulate in such rich deposits in the first place.
Piezoelectricity could also explain why gold veins seem to 'float' in seams of quartz, with no obvious cracks or variations in geochemistry to account for their arrangement. Nothing but the whisper of a mineral lightning storm left by the trembling Earth to show gold dust where to gather.
This research was published in Nature GeoScience.
