Australian researchers have developed a new device that will make surgery for chronic back pain safer and more affordable - and more importantly, much less invasive and painful. Which is a pretty big deal when you consider the fact that chronic back pain is the most common cause of pain and disability in people under the age of 50.
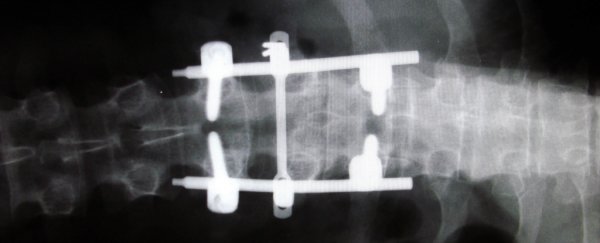
The new spinal fusion device is known as Thru-Fuze, and it works by holding the vertebrae of the spine in place to prevent movements that cause severe pain. But unlike current spinal fusion options, it doesn't require painful bone grafts to be taken, plus there's no need for screws or a cage system to be drilled into the spine.
Spinal fusion isn't a new idea. The surgery is already being performed on patients with severe back pain who can't be treated with medication, injections, or physical therapy. This is often caused by severe spinal degeneration or degenerative disc disease. But full fusion isn't achieved in more than 50 percent of these traditional procedures, and it can take up to a year to find out if the surgery has been a success.
"Existing methods of spinal fusion use rod or cage systems that require screws to be drilled into the spine and a painful bone graft harvested, which is the material used to form the bridge and obtain the fusion between the vertebrae in the spine," said inventor Bill Walsh from University of New South Wales (UNSW) Medicine.
"These systems are very costly, difficult and time consuming to implant and they also have relatively variable rates of fusion success."
Instead, Thru-Fuze is simply placed on the spine - kind of like a double-ended wrench with holes in it - and the fusion occurs as the bone grows through and on the device, locking everything in place. Testing in the lab and on animal models has shown that this happens shortly after surgery.
 UNSW
UNSW
"Over time, the device then acts as a bridge between the adjacent vertebrae for additional bone to grow across, fusing the adjacent vertebrae together, bone to bone," said Walsh.
The team has secured funding to conduct human trials, which are expected to begin at the Prince of Wales hospital in Sydney by the end of next year. And it's pretty exciting, as many medical inventions never make it out of the lab and into hospitals. But we're glad that this one did, as the device has the potential to help a whole lot of people, and potentially greatly reduce the global burden of disease.
"New technologies such as the Thru-Fuze are of paramount importance, as surgeons strive to deliver better patient outcomes with less invasive and more effective implant and prostheses options," said Ralph Mobbs from UNSW's Prince of Wales Clinical School, who will lead the patient trials.
Find out more below:

UNSW Medicine is a sponsor of ScienceAlert. Find out more about their world-leading research.