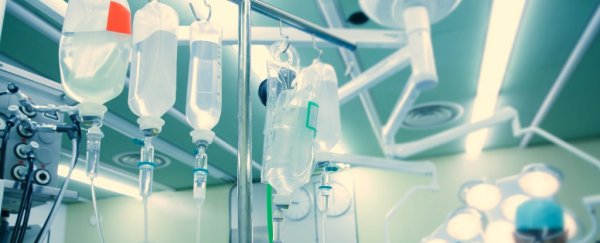
The US has a drug shortage problem.
In the wake of Hurricane Maria, a number of facilities that make healthcare products including intravenous fluids that are commonly used in hospitals have been knocked out of service.
That's led to shortages at hospitals around the US that are getting worse, according to the American Hospital Association, which represents thousands of hospitals and health systems.
"We are concerned that the shortages of widely-used and critical products are quickly becoming a crisis and looming threat to the public's health," Thomas Nickels, executive vice president of government relations and public policy at the AHA, wrote to the FDA.
The American Society of Health-System Pharmacists (ASHP), an organisation that monitors drug shortages, lists 139 compounds that are currently facing shortages.
In particular, hospitals are feeling the pain over a few commonly used IV fluids, including 50 and 100 milliliter injection bags of sodium chloride (also known as saline) at .9 percent, dextrose 5 percent, and nutritional solutions. All of these fluids are key in keeping patients hydrated and energised.
To get around the shortage, the AHA said, hospitals are using pill versions when possible, switching to versions that can be injected with just a syringe, and prioritising some patients over others.
How drug shortages happen
The ASHP cites a number of reasons for the shortages. Most are related to manufacturing problems. In the cases of saline and dextrose, Baxter's facilities in Puerto Rico were hit by the hurricanes, adding to existing drug shortages.
In other cases, some of the companies which make large portions of the drug simply stop making it, or a drug is only being produced by a single manufacturer.
And therein lies the problem: There simply are not enough companies making the drug to keep up with demand.
It's all part of a consolidation of the manufacturers who produce generic drugs.
US generic companies have had a harder time turning a profit on generic drugs while competing with companies outside the US that are able to make the same drugs at a cheaper cost.
That's caused manufacturers to home in on certain generic drugs and discontinue others that don't make as much money.
And if a generic manufacturer has a shortage, there's no easy fix - you can't just pass off the job to another company while the first fixes its problems, since getting approval to take on a new drug can take years.
When it comes to this particular shortage, the AHA said in its letter that it would like the FDA to push manufacturers that make these drugs to invest in creating more supplies in the future, as well as find suppliers within the US that aren't as susceptible to natural disasters.
This article was originally published by Business Insider.