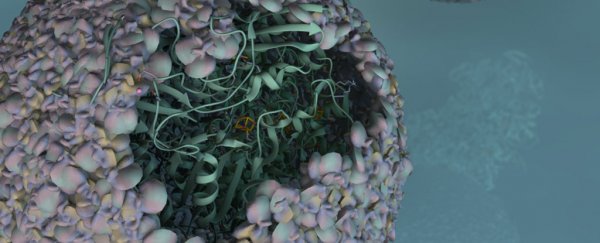
Researchers in the US have developed a virus-like biomaterial that catalyses the formation of hydrogen inexpensively and cleanly, which could lead to new environmentally friendly ways of producing biofuel.
The biomaterial is produced by placing a modified enzyme inside the protective protein shell of a virus, called a capsid. The resulting 'nano reactor' catalyst is 150 times more efficient than the unaltered form of the enzyme.
"Essentially, we've taken a virus's ability to self-assemble myriad genetic building blocks and incorporated a very fragile and sensitive enzyme with the remarkable property of taking in protons and spitting out hydrogen gas," said Trevor Douglas, a chemist at Indiana University. "The end result is a virus-like particle that behaves the same as a highly sophisticated material that catalyses the production of hydrogen."
To create the biomaterial, known as "P22-Hyd", the team co-opted two genes from the common bacteria Escherichia coli. These genes, called hyaA and hyaB, encode key subunits of the enzyme, hydrogenase. The enzyme is then inserted into the protective capsid of a bacterial virus known as bacteriophage P22.
The resulting fusion of hydrogenase and a virus shell is not only vastly more efficient at catalysing the production of hydrogen – it's also easy to create, requiring only a simple fermentation process. And compared to other means of creating fuel cells, such as using platinum to catalyse hydrogen, it's cheap and won't take a toll on the environment.
"This material is comparable to platinum, except it's truly renewable," said Douglas. "You don't need to mine it; you can create it at room temperature on a massive scale using fermentation technology; it's biodegradable. It's a very green process to make a very high-end sustainable material."
In addition to breaking the chemical bonds of water to produce hydrogen, P22-Hyd also catalyses the reaction the other way around, recombining hydrogen and oxygen to generate power.
The form of hydrogenase the researchers are using – NiFe (nitrogen-iron) – is one of only three occurring in nature, but it's the most suited to the task, easily integrating into biomaterials and tolerating exposure to oxygen. In its altered, protected form, the enzyme is vastly superior to unaltered NiFe enzymes thanks to greater resistance to heat and chemicals in the environment.
"No one's ever had a way to create a large enough amount of this hydrogenase despite its incredible potential for biofuel production," said Douglas. "But now we've got a method to stabilise and produce high quantities of the material – and enormous increases in efficiency."
The research has been reported in Nature Chemistry.
Update: We've amended the headline of this story to clarify that bacteria genes are inserted in the virus shell, not bacteria itself.