By heating carbon to an intimidating 1,000 degrees Celsius (1,800 degrees Fahrenheit), scientists have discovered a brand new elemental form that's ultra-strong and ultra-light, but also elastic like rubber and electrically conductive.
This new form of carbon not only offers up a range of extraordinary properties - the method used to find it could lead to the discovery of entire classes of materials we've never seen before.
As the fourth most abundant element in the Universe, and the second most abundant in our bodies (after oxygen), carbon isn't just the key component of much of life on Earth.
When it comes to its physical properties, there are few elements as diverse as carbon.
Certain atomic configurations will result in the soft, slippery form of graphite, but arrange it another way, and you'll get diamond - one of the hardest materials on the planet. And then there's graphene, which is the strongest material known to science.
Now, researchers have figured out that if you heat the element to almost 1,000 degrees Celsius, and put it under 250,000 times normal atmospheric pressure, you can produce an ultra-strong, yet super-flexible form of carbon that could see use in everything from exoskeletons to spaceships.
"Light materials with high strength and robust elasticity like this are very desirable for applications where weight savings are of the utmost importance, even more than material cost," says one of the team, Zhisheng Zhao from Yanshan University in China.
In the past, scientists have tried to create something like this, but without the right combination of heat and pressure, it would keep reverting back to its original structure.
Now, Zhao and his team have finally found the optimal conditions, which force the carbon to buckle, merge, and connect in a range of configurations.
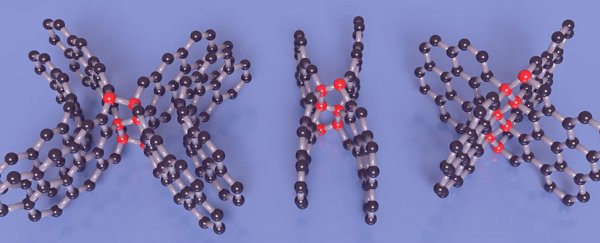
The result is a new, exotic type of carbon that contains both graphite-like and diamond-like bonds, plus layers of graphene, which help to impart softness and strength at the same time.
In the image below, you can see three different forms it can take, with the red spheres representing the diamond-like links, and the black spheres representing the layers of graphene:
 Timothy Strobel
Timothy Strobel
The material is now known as 'compressed glassy carbon'.
"The compressed glassy carbons have extraordinary specific compressive strengths - more than two times that of commonly used ceramics - and simultaneously exhibit robust elastic recovery in response to local deformations," the team reports.
Its elasticity beats organic rubber, silica, and even this shapeshifting titanium-nickel wire, and it's about five times stronger than common metals and alloys.
We'll have to wait and see which industries will take an interest in this new material, but in the meantime, the researchers expect the same technique can be used to find a whole range of never-before-seen materials.
"[W]e believe that this synthesis method could be honed to create other extraordinary forms of carbon and entirely different classes of materials," says Zhao.
The research has been published in Science Advances.