A common, widespread fungus that's probably hiding in your garden soil right this moment is capable of transforming into a formidable predator when it gets too hungry.
Scientists have known about the hunting abilities of Arthrobotrys oligospora since at least the 1980s, but they're still in the process of figuring out exactly how this otherwise peaceful fungus transforms into a worm's worst nightmare.
Now, a research team from Taiwan and the US led by molecular biologist Hung-Che Lin from Academia Sinica in Taipei, has unraveled some of the strategies the fungus uses to trap and consume its prey.
Usually, A. oligospora survives on organic matter that's already dead. But when nitrogen supplies become scarce, it does what it must to survive.
This fungi is just one of several that can trap and kill prey when necessary.
"Different trapping devices, including adhesive nets, columns, knobs, non-constricting and constricting rings, are formed depending on the fungal species," the authors write.
Predator mode is only fully activated when the fungus senses roundworms are nearby.
Previously, Lin was on a team that identified the specific gene pathway that gives A. oligospora its ability to 'smell' worm pheromones. This new research tracks the molecular frenzy that follows.
They found that once the fungus detects its prey, DNA replication and ribosome production increases, which in this case, is a bit like the microbial equivalent of researching and gathering equipment in preparation for the hunt.
In the next stage, researchers saw an increase in the activity of genes involved in building and operating worm traps.
"Among all the time points sampled, we observed the greatest differential expression (both up- and down-regulation) at 10 hours post exposure, which corresponds to a period of intense trap formation and adhesion between fungal and nematode cells," they write.
They identified a new class of proteins on the trap's surface they've called trap enriched proteins (TEP), which were found to be critical for trap adhesion to nematodes.
In fungi where this protein was deactivated, only 10 percent of nematodes placed on the fungi were caught after 10 minutes – a drastically lower hit rate than the 100 percent rate for intact traps measured during the experiment.
Another known protein involved in this stage syntaxin, is involved in transporting 'worm adhesive' – a kind of natural 'glue' that oozes from the trap, making it too sticky for the prey to wiggle free. When this protein was deleted from the fungi, 70 percent of nematodes were able to escape the mutant traps, compared with almost no misses for wild-type fungi.
Once the fungus traps its prey, it penetrates the worm's body and digests it using filaments called hyphae. Instead of eating by chewing and swallowing like we do, this hyphae network fills the worm from the inside to break down and absorb nutrients for transport to wherever they're needed.
During this stage, the researchers saw a spike in activity of the genes that encode for protease enzymes. Proteases are crucial for digestion – in humans, they are produced in the stomach, pancreas, and small intestine. The flurry of protease-related gene activity in A. oligospora's successful traps suggests the fungus is using these enzymes to help digest their prey.
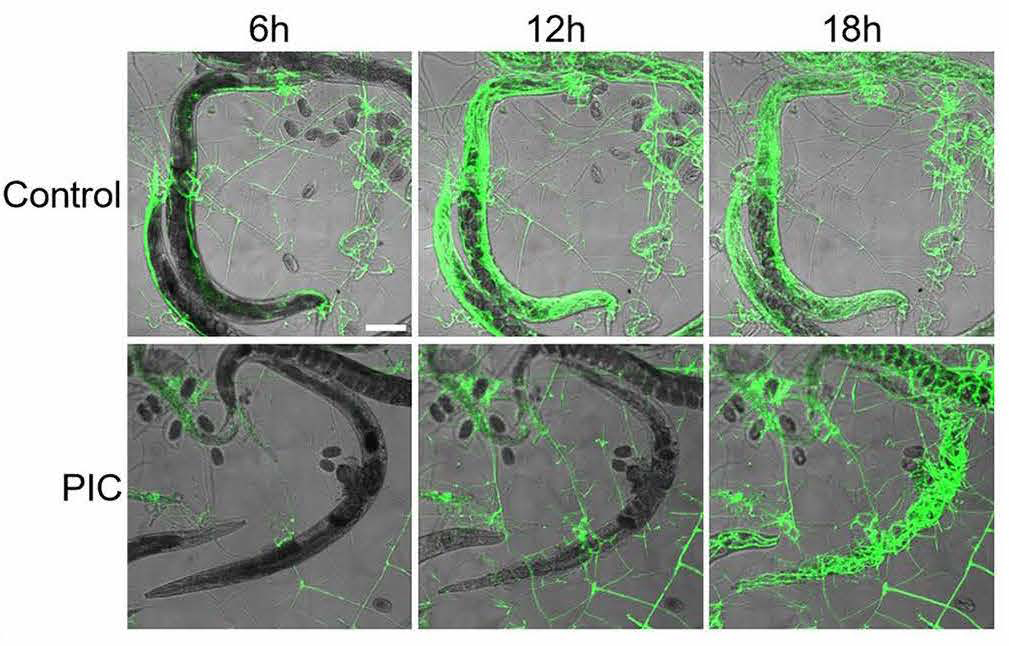
To understand the role of protease in digesting nematodes, the researchers gave some fungi a protease-inhibiting cocktail. Twelve hours after nematode exposure, it was obvious the proteases were indeed playing a significant role, particularly in how quickly and efficiently the worm was digested, which was far less for those given the cocktail.
Another experiment tested the impact of deleting the genes for certain proteases – mutant fungi were still able to trap and digest their prey, but there were minor defects in the way the hyphae colonized the worm's carcass.
"Our comprehensive transcriptomics and functional analyses highlight the role of increased DNA replication, translation, and secretion in trap development and efficacy," the authors explain.
"These results furthered our understanding of the key processes required for fungal carnivory."
The research is published in PLOS Biology.