Scientists have calculated the molecular form of a new kind of ice to find that it could have the lowest density for frozen water ever discovered.
If it can be synthesised, the proposed ice – which does not naturally occur on Earth – would become the 18th known crystalline form of water. However, due to a naming quirk over the first two identified forms, it would go by the name 'Ice XVII'. Who outside the world of experimental chemistry would ever have guessed there were so many ices?
"We performed a lot of calculations (focused on) whether this is not just a low-density ice, but perhaps the lowest-density ice to date," said chemist Xiao Cheng Zeng from the University of Nebraska-Lincoln. "A lot of people are interested in predicting a new ice structure beyond the state of the art."
To do just that, the researchers used a computational algorithm and molecular simulation to predict their new molecular form of frozen water. If it can be synthesised, the ice will be about 25 percent less dense than the current lowest-density record-holder, developed by European researchers in 2014.
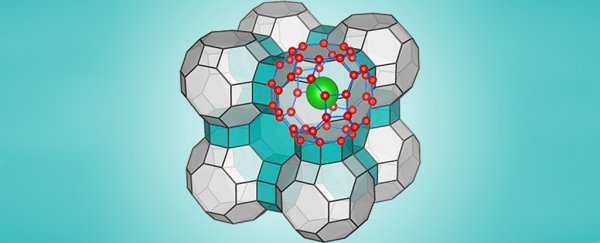
The simulation helped the scientists determine the ranges of extreme pressure and temperature that would be required to freeze water in their predicted form. That configuration is a type of clathrate – a series of molecules, in this case water molecules, that create an interlocking cage-like structure.
While it was once believed that these kinds of clathrate structures could only maintain their structural integrity if they contained a 'guest molecule' within them, scientists are now calculating that these cages can hold together even once such a trapped chemical exits the surroundings.
Which isn't to say synthesising 'Ice XVII' will be easy. According to the researchers' calculations, the ice will only form in this configuration if water molecules are placed inside an enclosed space that is subjected to extremely high, outwardly expanding pressure – although the exact amount required depends on the temperature involved.
At –23.3 degrees Celsius (–10 degrees Fahrenheit), the amount of pressure needed to produce the ice would be greater than the pressure at the Pacific Ocean's deepest trench, the researchers say. Colder temperatures would require greater pressure, with –273.3 degrees Celsius (–460 degrees Fahrenheit) equating to the amount of pressure you'd feel if you were trapped under 300 jumbo jets at sea level.
With these levels of pressure, you could synthesise the ice by vacuuming out the guest molecules. That's the next step for the researchers, who, if successful, will have created the first new kind of ice discovered in the US since before World War II.
"Water and ice are forever interesting because they have such relevance to human beings and life," said Zeng. "If you think about it, the low density of natural ice protects the water below it; if it were denser, water would freeze from the bottom up, and no living species could survive. So Mother Nature's combination is just so perfect."
The findings are reported in Science Advances.