When one cell divides into two, a tiny parcel of cellular stuff is left behind, and scientists have just realized how its contents could transform neighboring healthy cells into fast-dividing cancerous ones.
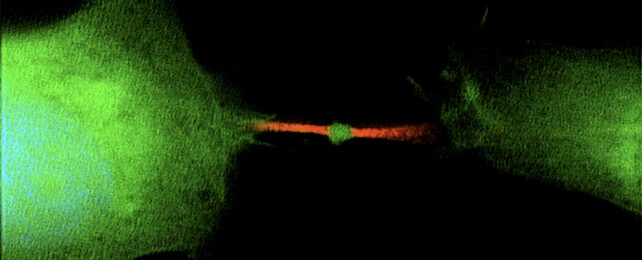
Called a midbody remnant, the parcel is a millionth of a meter in size and stuffed with genetic instructions for dividing cells.
Researchers had thought of midbody remnants as the garbage bag of cell division, once considering them to be dissolved post-split, before noticing they were instead expunged.
But a new study has tracked the formation of these overlooked organelles and unpacked their internal structure, which suggests they are well-equipped to influence other cells after their primary job of cell division is done.
"What surprised us is that the midbody is full of genetic information, RNA, that doesn't have much to do with cell division at all, but likely functions in cell communication," says geneticist and senior author Ahna Skop at the University of Wisconsin-Madison.
Before we get to what midbodies do, let's first understand how they form.
When two body cells divide in a process called mitosis, a whole lot of internal shuffling takes place. Chromosomes duplicate and line up down the midline of the cell, which sprouts spindly microtubules to drag one copy of each chromosome in different directions to create two new daughter cells.
The midbody assembles itself at the overlapping, outstretched ends of spindle microtubules that tether themselves to chromosomes. From there, the midbody recruits and positions the abscission machinery that cleaves the final bridge connecting the dividing cells in two.
Job done; midbody remnants were thought to be degraded inside daughter cells. But studies revealed that most of these parcels are actually released into extracellular space and ingested by other neighboring cells.
What researchers found next began to reveal the true nature of midbody remnants, as cell signaling packages, not trash cans.
Both cancer cells, which divide unchecked, and stem cells, which are plastic precursors that develop into other cell types, gobble up midbody remnants more so than regular cells.
Studies have also found cells that engulf midbody remnants become more invasive, suggesting a role for midbody remnants in cellular reprogramming and proliferation.
But this is an emerging field, and the cell signaling mechanisms of midbody remnants are only just beginning to be understood. So Skop and her colleagues wanted to delve deeper into the contents of these tiny cargo carriers.
"Here, we further define the structural components, organization, and behavior of midbodies throughout their uniquely complex life cycle," the team writes in their published paper.
Skop and colleagues found that midbody remnants were loaded with certain types of RNA: those encoding proteins involved in cell proliferation, pluripotency (the ability of stem cells to develop into any cell type), and oncogenesis (where healthy cells become transformed into cancerous ones).
What's more, the midbody remnants also contained the cellular machinery necessary to turn that RNA into functional proteins. As the midbody remnant is released, that machinery is busy translating the RNA into proteins, as if the capsule is getting ready for its next task: intercellular communication.
"It's like a little lunar lander," Skop says of midbody remnants. "It's got everything it needs to sustain that working information [away] from the dividing cell."
More work is needed to comprehensively track the fate of these surprise-filled packages to figure out how long they last adrift in extracellular space and how far they travel before being taken up by other cells.
If their RNA-encoded messages are enacted, they might well coax other cells into dividing more rapidly than they should. But cancer is an accumulation of many errors, and there are plenty of mechanisms to keep unruly cells in check.
The study has been published in Developmental Cell.