Just when scientists thought they had almost figured out the origins of multicellular life, evolution throws another curveball.
In a serendipitous discovery, a team of researchers has just chanced upon a third type of 'unconventional' multicellularity that blends the two kinds we already knew about.
Multicellularity has evolved a staggering 45 times or more across the tree of life. Yet fundamentally, the ancestor of each multicellular lineage relied on just one of two methods - individual cells sticking together as they split, or individual cells that have previously split coming back together.
Clonal multicellularity, where cells remain connected as they divide over and over again, gives rise to organisms as simple as filamentous cyanobacteria, and as complex as animals with their specialized tissues.
The other kind of multicellularity, called aggregation, is a temporary clustering of free-living cells, which happens with eerily cooperative slime molds and social amoeba, often in response to harsh environmental conditions or predation.
Núria Ros-Rocher, an evolutionary biologist at the Pasteur Institute in France, and colleagues made their surprise discovery when studying choanoflagellates: single-celled, water-dwelling organisms that form short-lived colonies and represent the closest living unicellular relatives of animals.
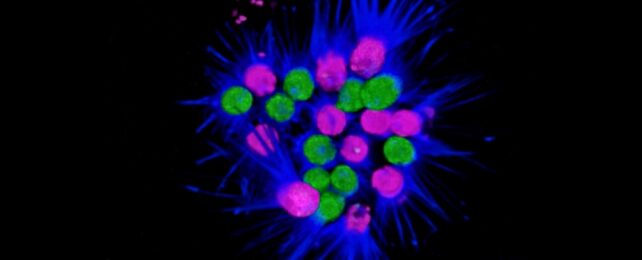
Specifically, the team was observing the behavior of a choanoflagellate called Choanoeca flexa. This species forms spectacular colonies that look like fireworks exploding, which could help us better understand gastrulation, a critical step in embryonic development.
Everywhere scientists have looked, choanoflagellates formed colonies via cell division, replicating themselves to increase their assembled numbers.
So nothing was out of the ordinary when Ros-Rocher and colleagues saw cells dividing inside C. flexa colonies, which were first discovered in 2018 in splash pools on the rocky coast of the Caribbean island of Curaçao.
However, under the microscope in the lab, the researchers also noticed that C. flexa colonies reformed very fast after being dissociated with light forces – too fast to be down to cell division.
Then, when the researchers used fluorescent dyes to stain different types of C. flexa cells, they could see distinct single-cell populations forming dual-labeled chimeric clusters – which would not be possible if they had divided from the same founder cell.
At first, the team questioned their strange observations of C. flexa colonies because they seemed "too weird", senior author and Pasteur Institute cell biologist Thibaut Brunet explained in a thread on social media.
But now, based on the findings of their imaging experiments, the team thinks C. flexa can aggregate into single-layer, sheet-like colonies, like slime molds, or develop clonally, like animals do.
"We find C. flexa sheets can form through purely clonal processes, purely aggregative processes, or a combination of both, depending on experimental conditions," the team writes in a preprint posted to the bioRxiv preprint server ahead of peer review.
"Making use of both mechanisms at once – mechanisms often depicted as mutually exclusive – likely allows sheets to develop faster than they would by a single mechanism acting alone," Brunet adds.
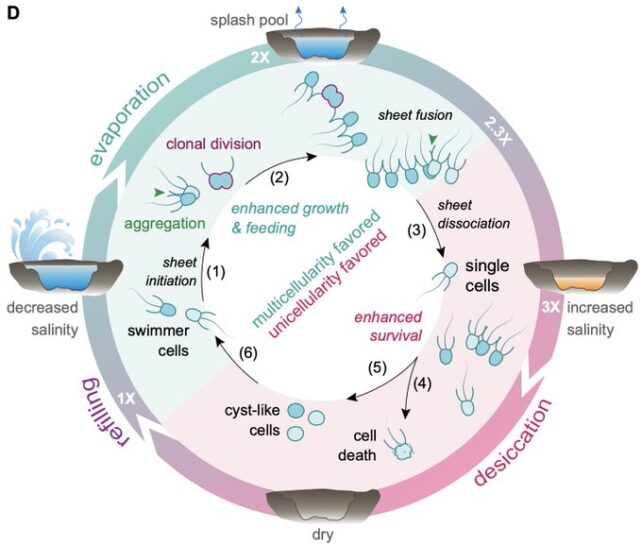
Further experiments showed that C. flexa's multicellular form depended on the conditions of the shallow seawater pools in which the organisms were found.
When the salinity of these tidal pools crossed a critical threshold, tripling as the seawater evaporated, C. flexa colonies dissociated into single cyst-like cells to resist drying out. When rehydrated in the lab, C. flexa reformed colonies within a few days, aggregating first and then dividing.
"We think we found an environmentally entrained life cycle: evaporation-refilling cycles causing fast transitions into and out of multicellularity," Burnet says.
While the research is yet to be peer-reviewed, and so its findings may be queried, discovering this unusual mixed mode of development "expand[s] the range of possible scenarios for the emergence of animal multicellularity," the team concludes.
The study is available on bioRxiv.