Eavesdropping on the earliest conversations between tissues in an emerging life could tell us a lot about organ growth, fertility, and disease in general. It could help prevent early miscarriages, or even tell us how to grow whole replacement organs from scratch.
In a monumental leap in stem cell research, an experiment led by researchers from the University of Cambridge in the UK has developed a living model of a mouse embryo complete with fluttering heart tissues and the beginnings of a brain.
The research advances the recent success of a team comprised of some of the same scientists who pushed the limits on mimicking the embryonic development of mice using stem cells that had never seen the inside of a mouse womb.
In the past, researchers in embryology have focused largely on plucking choice stem cells from parts of an embryo that would grow into an animal and encouraging them to proliferate in glassware full of specially selected nutrients.
Over the years, this method has resulted in clumps of cells containing the basic starting structures of a gut and a fold of tissues called the neural tube.
What the so-called 'gastruloid' model contains in form, however, it lacks in function. Many features expected to develop alongside these tissues aren't present, making it harder to draw parallels between the model and an authentic growing embryo.
There are ways to encourage brain-like structures to appear, as well as functioning heart tissue and a more complex gut tube. Yet workarounds based on comparatively simple hormonal soups can only go so far.
Be it mouse or moose, or humans or horses for that matter, placental mammals all start life in roughly the same way. Shortly after fertilization, the first cell splits until there are three basic domains of tissue: one that proceeds to create the animal itself, and two that contribute to organs that facilitate its growth inside the mother.
Where the first can generate a model embryo (or embryoid) on its own, having the second two groups of placental cells nearby provides the necessary chemical negotiations that promote a litany of tiny changes in the developing animal.
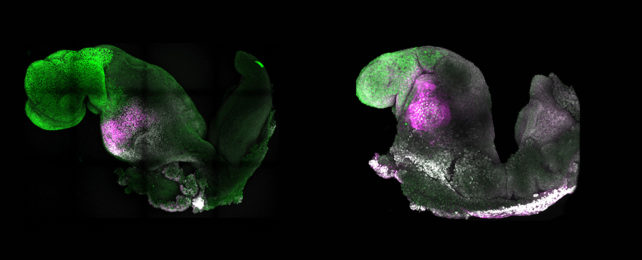
Mixing stem cells representative from these three major tissue groups and improving on previous methods for their development in vitro (that means in a dish) into an embryoid, the team found their model could progress under its own steam to develop a nervous system equivalent to a natural mouse embryo at 8.5 days post-conception.
The step is a small one, equivalent to just a single day of development for an unborn mouse. But a lot can happen in that 24 hours of gestation.
The synthetic embryoid also contained foundational heart tissue that twitched out a beat and the beginnings of a gut, as well as the start of structures that in an actual embryo could build parts of the skeleton, muscles, and other tissues beneath the skin.
On its own, the model wouldn't continue to develop into anything like a thriving baby mouse. Science is far from able to produce anything so advanced as a functional organ from stem cells alone, let alone an entire animal.
While the resemblance is quite significant in research, it is – so to speak – only skin deep, lacking the signals that would see it transform into the fully-formed organism it models.
Having a collection of tissues that authentically reflects development outside of a body provides researchers with the opportunity to not only observe, but ethically test genetic changes that could help improve our understanding of how our bodies grow.
"This period of human life is so mysterious, so to be able to see how it happens in a dish – to have access to these individual stem cells, to understand why so many pregnancies fail and how we might be able to prevent that from happening – is quite special," says Magdalena Zernicka-Goetz, a developmental biologist at the University of Cambridge in the UK.
The researchers note there is already evidence of ways they can further optimize the process to better emulate natural development.
Of course, the philosophical question of when a 'close copy' becomes 'too close' is one UK legislators are already discussing, limiting growth of human embryos in the laboratory to just 14 days.
With future research aiming to swap out mouse cells in order to advance existing human embryoid development, it's a challenging question that is bound to be pulled apart.
It's an important balance to strike, given the rewards at stake, even in fields outside of embryology.
"There are so many people around the world who wait for years for organ transplants," says Zernicka-Goetz.
"What makes our work so exciting is that the knowledge coming out of it could be used to grow correct synthetic human organs to save lives that are currently lost."
The research was published in Nature.