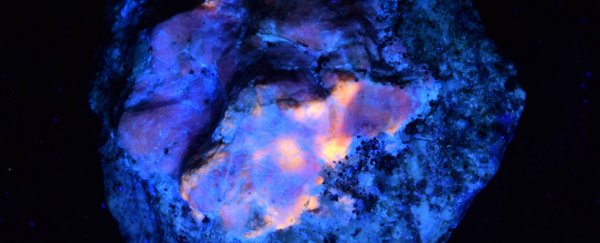
The afterglow of the mineral hackmanite (or tenebrescent sodalite) is a fascinating natural phenomenon that has long been a mystery to scientists – even if we're now able to engineer synthetic materials that glow in the dark more effectively than anything in nature.
Geologists first described the mineral in the 1800s, who were intrigued by its tendency to softly glow a bright pink hue when broken or placed in the dark and lose it in the light. Later research would narrow down the chemistry behind this characteristic, but the precise nature of the reaction has proven elusive.
Now a new study outlines exactly how certain types of hackmanite retain some of their glow as they move from bright to dark settings. The key is the delicate interplay between the mineral's natural impurities, determined by how it was formed.
Getting a better understanding of how hackmanite can emit white luminescence in dark conditions will further help scientists develop our own synthetic materials able to glow in the dark without any source of power, as on an emergency exit sign, for example.
"We have conducted a lot of research with synthetic hackmanites and have been able to develop a material with an afterglow distinctly longer than that of natural hackmanite," says materials chemist Isabella Norrbo from the University of Turku in Finland.
"However, the conditions affecting the luminescence have been unclear so far."
A combination of both experimental and computational data was studied to determine that the concentrations and balance of sulfur, potassium, titanium, and iron were most important when it came to the afterglow given off by hackmanite.
In particular, titanium was found to be the element actually glowing, with the glow itself powered by electron transfer.
However, titanium concentrations alone are not enough to create luminescence, with the right mix of other elements also required.
The researchers say that synthetic materials can be improved and made more efficient and reliable through these sorts of studies – even if nature isn't able to match the strength of the glows that can be engineered in the lab.
"The materials used at the moment are all synthetic, and, for example, the material with the familiar green afterglow obtains its glow from an element called europium," says materials chemist Mika Lastusaari, from the University of Turku.
"The difficulty with this kind of material is that even though the desired element that emits luminescence can be added to them, their afterglow properties cannot be predicted."
Samples of hackmanite from Greenland, Canada, Afghanistan, and Pakistan were used in the study, with an international team of chemists, mineralogists, geologists, physicists, statisticians, and other scientists involved in working out exactly what was happening with the hackmanite glow.
Part of the mystery was why some hackmanites show a glow and others don't, but through a careful comparison of the different samples, the team was able to spot the required mix of orange photoluminescence (turning absorbed photons into light), blue persistent luminescence (emitting light without heating), and purple photochromism (a form of chemical transformation caused by electromagnetic radiation).
It's a complex mix of natural elements and chemical reactions, but the result should be better synthetic materials that can match these sorts of glows. In terms of material science, it's important not just how bright the luminescence is but also how long it lasts.
"With these results, we obtained valuable information of the conditions affecting the afterglow of hackmanites," says Lastusaari.
"Even though nature has not, in this case, been able to form a material with a glow as effective as in synthetic materials, nature has helped significantly in the development of increasingly more effective glowing materials."
The research has been published in the Chemistry of Materials.