Organ transplants are an invaluable way of saving people's lives when their own organs fail, but organ shortages, waiting lists, and the powerful drugs required to help recipients' bodies accept their new parts are just some of the difficulties with existing transplant processes.
But what if there were another way of replacing organs, one that was less reliant on sourcing whole, living organs from other people's bodies? Scientists in the US have made progress towards creating bioengineered human hearts in the lab, by regenerating a functional human heart muscle. In this case, the procedure still requires using a donated organ, but one that's fused with cells from the recipient.
The technique involves repopulating a decellularised organ – stripped of the original donor's living cells – with new cardiac tissue grown from the potential recipient's induced pluripotent stem cells ( iPSCs). In effect, the donor heart is stripped of the components that would trigger an immune response from the recipient, and is replaced with the recipients' own cardiac muscle cells.
"Regenerating a whole heart is most certainly a long-term goal that is several years away, so we are currently working on engineering a functional myocardial patch that could replace cardiac tissue damaged due [to] a heart attack or heart failure," said researcher Jacques Guyette from the Massachusetts General Hospital Centre for Regenerative Medicine (CRM).
The study, documented in Circulation Research, was led by CRM surgeon Harald Ott, who previously developed a decellularisation procedure to strip living cells from rat organs with a detergent solution, before repopulating them with organ-appropriate grown cells. In the new study, this multi-stage process has been scaled up and conducted on human hearts for the first time.
 Bernhard Jank, MD, Ott Lab, Centre for Regenerative Medicine, Massachusetts General Hospital
Bernhard Jank, MD, Ott Lab, Centre for Regenerative Medicine, Massachusetts General Hospital
"Generating functional cardiac tissue involves meeting several challenges," said Guyette. "These include providing a structural scaffold that is able to support cardiac function, a supply of specialised cardiac cells, and a supportive environment in which cells can repopulate the scaffold to form mature tissue capable of handling complex cardiac functions."
In the study, which drew upon 73 human hearts authorised for scientific research, the researchers induced pluripotent cells to differentiate into around 500 million cardiac muscle cells (cardiomyocytes), then seeded them into the tissue of the decellularised hearts.
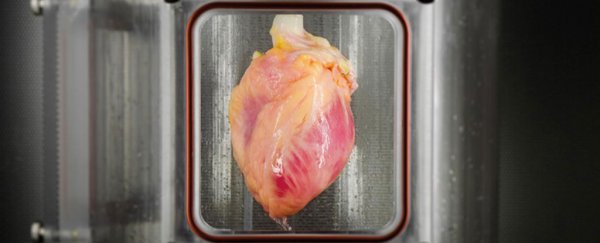
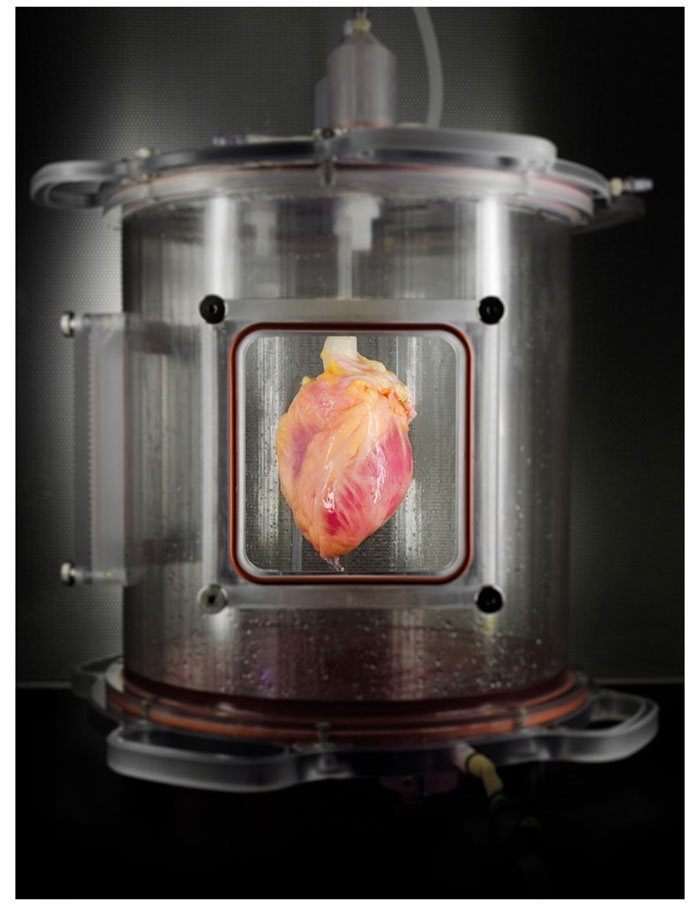
After several days in culture, the cardiomyocytes developed into spontaneously contracting tissue, which the researchers say represents the first regeneration of human heart muscle from pluripotent stem cells within a cell-free, human heart matrix. The beating organs were then mounted in an automator bioreactor system (pictured), which provides the muscle with a nutrient solution and reproduces certain conditions within a living heart.
The research might seem a bit grisly – the team is veering close to Re-Animator territory, after all – but the future applications for healthy, lab-grown organs hold a huge amount of promise.
"Among the next steps that we are pursuing are improving methods to generate even more cardiac cells – recellularising a whole heart would take tens of billions – optimising bioreactor-based culture techniques to improve the maturation and function of engineered cardiac tissue, and electronically integrating regenerated tissue to function within the recipient's heart," said Guyette.