Carbon can be arranged in a number of configurations. When each of its atoms is bonded to three other carbon atoms, it's relatively soft graphite. Add just one more bond and it becomes one of the hardest minerals known, diamond. Chuck 60 carbon atoms together in a soccerball shape and boom, buckyballs.
But a ring of carbon atoms, where each atom is bonded to just two others, and nothing else? That's eluded scientists for 50 years. Their best attempts have resulted in a gaseous carbon ring that quickly dissipated.
So it's a pretty big deal that a team of researchers, from Oxford University and IBM Research, has now created a stable carbon ring.
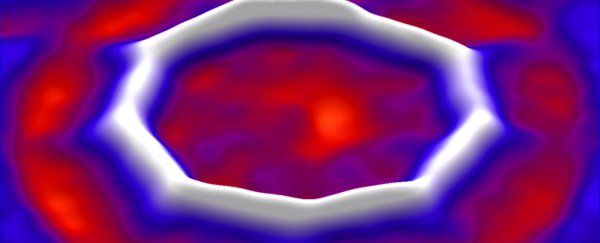
The ring-shaped carbon compound, called a cyclocarbon, is made from 18 carbon atoms, the smallest cyclocarbon that is predicted to be thermodynamically stable. And advanced microscopy techniques have provided images of it.
So far, research into the cyclocarbon's structure has suggested that it acts as a semiconductor, which means it has potential use in electronics. And the very property that made cyclocarbons so difficult to isolate in the first place - their high reactivity - means they could be used to create other carbon allotropes and carbon-rich materials.
To pull off this feat, the researchers started by synthesising the triangular cyclocarbon oxide C24O6. That's the 18 carbon atoms, bonded to six carbon monoxide molecules, two clustered at each of the three corners of the triangle.
They transferred this concoction to a layer of sodium chloride on a copper plate, chilled in a vacuum chamber to just above absolute zero. This provides an inert surface that keeps the structure stable.
Then, using the tip of an atomic force microscope, the team knocked the carbon monoxide (CO) molecules off the structure, leaving just the ring of carbon atoms behind.
 (IBM Research)
(IBM Research)
It sounds a lot easier than it was, of course. The researchers couldn't always knock all the CO off without collapsing the ring structure; in many cases, they instead produced molecules such as C22O4 and C20O2 (see image above).
"We removed all six CO moieties from C24O6, with 13 percent yield, typically resulting in circular molecules," the researchers wrote in their paper.
Interestingly, the atoms in the cyclocarbon formed what is known as a polyynic structure, with alternating triple and single bonds. It's long been a question whether one-dimensional carbon would produce this or a cumulenic structure, with repeated double bonds.
It's this alternating structure that is thought to produce semiconductivity - and it suggests that carbon chains would also be semiconductive.
Now what? Well, that's still a little unclear. The process of building the cyclocarbons will need to be refined in order to produce a more reliable yield, for a start. At the moment, the rings can only be built one at a time, too, so the team plans to find ways for building multiple cyclocarbons at once.
And now that stable cyclocarbons have been produced, scientists can start experimenting with applications - figuring out how the semiconductivity can be exploited, for instance, or exploring cyclocarbon properties as a basic building block for even more complex molecules.
"Our results," the researchers wrote, "provide direct experimental insights into the structure of a cyclocarbon and open the way to create other elusive carbon-rich molecules by atom manipulation."
The research has been published in Science.