Set aside every scrap of iron inside a human body and you might have enough to fashion a nail or two. As for copper, you'd be lucky to extract just enough to make a small earring.
Scarce as they are, these two metals are necessary for our survival, playing essential roles in human growth and metabolism. But one place we wouldn't expect to find either is clumped inside our brain cells.
However, for people with the neurodegenerative disorder Alzheimer's disease, something seems to be turning these elements into microscopic ingots.
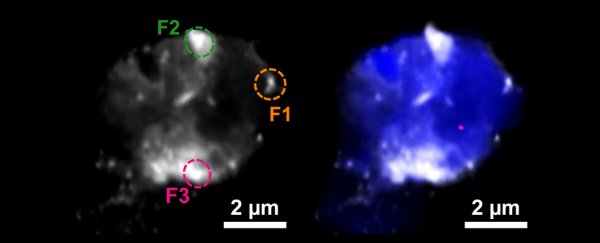
A team of researchers from the US and UK spotted the tell-tale glint of copper and iron in their elemental forms using a form of X-ray microscopy (STXM) on samples of neural plaques taken from the frontal and temporal lobes of Alzheimer's patients.
Plaques are a typical feature of this particular form of dementia, made up of proteins broken down into what's known as beta-amyloid.
Yet therapies focused on clearing clumps of beta-amyloid from the brain haven't led us much closer to a treatment for Alzheimer's, leaving researchers wondering what role – if any – they play in the disease's progress.
Ongoing research has continued to build a picture surrounding the biology that could be responsible for the plaques, with researchers looking at their formation and taste for destruction from every angle.
One angle that hasn't been fully explored is the toxic effect of biomineralization, or the accumulation of minerals such as hematite in brain cells.
Trapped as a charged ionic form inside hemoglobin, iron is a handy way to transport oxygen around the body. And few places are as desperate for oxygen as the human brain.
Once released from its protein shackles, however, iron shows its nasty side as what's known as its labile form, generating reactive species of oxygen that wreak havoc on delicate biochemistry and destroying cells.
High levels of labile iron have been linked to neurodegenerative disorders like Alzheimer's before. Similarly, copper is another mineral typically shielded safely in a protein, yet thoroughly capable of making a mess of our brains in labile form.
To build a bigger picture of how these two metals might be involved in dementia, a research team led by scientists from Keele University in the UK hunted for signs of iron and copper inside resin-embedded amyloid plaque cores donated by two Alzheimer's patients.
The X-rays the research team used to analyze the plaques allowed them to get a feel for the clumps' size and shape while also revealing the identity of the minerals they contained.
Not only did they spot accumulations of iron and copper, but these metals were in elemental forms, essentially forming tiny deposits buried deep inside the amyloid plaques. What's more, the team could tell a few things about the metals' ability to react with other substances.
Although in a relatively unreactive state and barely a fraction of a micrometer wide, the surfaces of these nuggets wouldn't be all that stable, posing a risk of reverting to hostile forms that could react and cause damage to the surrounding neuron.
With signs of these ionized states spotted near the nuggets, there's every reason to suspect they could well be playing a role in inflammation and even death of the cells.
Aggregations of iron as the magnetic mineral magnetite have been found previously in bacteria and a few other species of animal. They've also been found previously in post-mortems of human brain tissue.
But this is the first time anybody has uncovered tiny pieces of copper in elemental form inside human neurons.
Exactly what it means will depend on what future studies find. Even if it doesn't present a new pathway for treatment, it just might offer insight into ways to diagnose Alzheimer's even earlier or tease apart the different ways it might progress.
With the prevalence of the disease set to rise with the expansion of our world's aging population, understanding how Alzheimer's develops is becoming more important than ever.
We'll need every nugget of information we can get our hands on, no matter how small.
This research was published in Science Advances.