We've seen pig brains reanimated post-mortem and kept alive for hours. Now scientists from Japan have taken tiny slivers of mouse brain tissue, and kept it alive and viable for 25 days, isolated in a culture.
This has greatly increased the timeframe that isolated brain tissue can maintain its functions, from days to weeks - which in turn could vastly improve research into therapeutic drugs.
The key to the team's success was a new method of keeping tissue alive - which involved combining a special type of membrane with a modified microfluidic device.
Microfluidic devices use tiny channels for delivering fluid to tissues, and they have distinct advantages over regular culture dishes for ex vivo tissue experiments.
In addition to the precision of fluid delivery, they're more customisable, can mimic certain cell behaviours, and require smaller sample volumes, which makes it easier to study cell interactions.
But the thing about studying how systems in our bodies react to things is that a few days just isn't enough to see long-term effects. This is where our current methods using microfluidic devices for keeping these cellular systems alive fall short.
The problem is maintaining a balance. These tissues dry out quickly, so you need to keep the system flushed with moisture and nutrients with a wet culture medium; but too much moisture prevents the cells from exchanging the gases the tissue needs to survive, essentially drowning it.
And it's this problem that the team set out to solve with their new system.
 (RIKEN)
(RIKEN)
The device, as a supervillain would say, is genius in its simplicity. It consists of a semi-permeable microfluidic channel, surrounded by a permeable artificial membrane and solid walls. These solid walls are made of polydimethylsiloxane, an organosilicon polymer commonly used in microfluidic devices.
So, rather than the tissue sitting constantly in a bath of culture medium, the fluid circulated through the channel and passed through the permeable membrane to keep the tissue moist while still allowing gases to be exchanged between the cells.
However simple it sounds, though, the researchers said it was not so easy to pull off.
"Controlling the medium flow was difficult because the microchannel that formed between the PDMS walls and the porous membrane was unusual," said biochemist Nobutoshi Ota of the RIKEN Center for Biosystems Dynamics Research.
"However, we had success after trial and error modifications to the porous membrane and adjustments of the inlet/outlet flow rates."
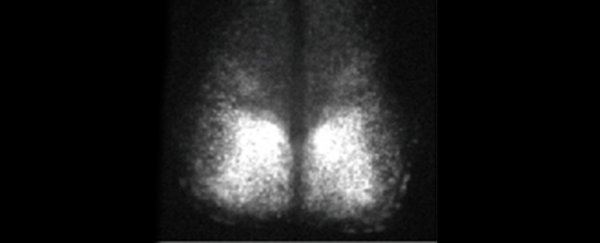
The team used a tiny piece of brain called the suprachiasmatic nucleus (SCN), responsible in mammals for maintaining the circadian clock and biological rhythms. The neuronal cells in the SCN exchange and synchronise phase information by moving peptides and small molecules between cells, which makes the SCN ideal for studying cell interactions.
The mice they harvested these SCNs from had been genetically edited so that circadian rhythm activity in the brain was linked to the production of a fluorescent protein; so when all is working as it should, the tissue fluoresces.
 (Ota et al., Analytical Sciences, 2019)
(Ota et al., Analytical Sciences, 2019)
And fluoresce it did, for 25 days, the researchers said, compared to their control of the same tissue in a more conventional culture dish. After 10 hours, activity in the control had already declined by 6 percent.
And the only reason the tissue in the experimental system only lasted 25 days is because that was the cut-off time for the experiment. The researchers expected that it could have lasted over 100 days.
That is what they plan to try for their next experiment. They believe that it could be used for all organ tissues, not just brains. And there's potential for lab-grown human organs, too.
"This method can be used for more than explanted tissues from animals," Ota said.
"It will also improve research into organogenesis through long-term culturing and observation which is necessary for growing tissue and organs."
The research has been published in Analytical Sciences.