There's nothing simple about repairing spinal cord injuries. But new research has pinned down how one of the most cutting edge techniques works, and in particular how the body can repair itself with a little prompting from surgeons.
As well as giving experts more insight into existing treatments, it's hoped the study will lead to techniques for tackling other types of damage to the nervous system – perhaps even in cases where the spinal cord itself is severed.
The team from King's College London in the UK focussed on a recently developed method for reconnecting sensory neurons to the spinal cord after traumatic injuries, looking at how the repair happens on a cellular level, and the way in which small neural offshoots grow to reconnect broken circuits in the body.
"The strategy of encouraging new growth from spinal neurons could potentially be of use in other injuries of the nervous system," says one of the researchers, Thomas Carlstedt.
The spinal cord handles both motor neurons for muscle movement, and sensory neurons for pain, touch, and so on, enabling all the body's nerve cells to communicate with the brain.
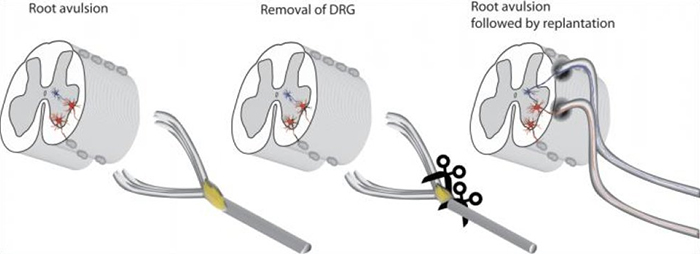 A new medical technique places torn roots deeper in the spinal cord. Credit: Thomas Carlstedt and Mårten Risling
A new medical technique places torn roots deeper in the spinal cord. Credit: Thomas Carlstedt and Mårten Risling
Where these two types of neurons connect with the spinal cord you get bundles called motor roots or sensory roots. In traumatic injuries, these roots get torn, causing a loss of connection between the parts of the body.
Motor roots can usually be replanted and regrown by surgeons, but sensory roots have been much trickier to rebuild until the development of the new technique being studied here.
"Doctors previously considered this type of spinal cord injury impossible to repair," says one of the team, Nicholas James. "These torn root injuries can cause serious disability and excruciating pain."
The new method involves cutting out the original sensory nerve cells from the root and planting the root deeper in the spinal cord, in an area called the dorsal horn, which is filled with more sensory neurons that don't normally connect directly to sensory roots.
When tried in patients, certain spinal reflexes returned, showing that some of the neural circuits had been reconnected.
But how? That was the question behind the new study, which replicated the same injuries and repair techniques in rats, using electrical pulses to see how the neural circuits had managed to fix themselves.
Analysis showed small neural offshoots had sprouted from dendrites in the dorsal horn, tiny branched projections at the end of neurons – they'd essentially reached out to the implanted sensory root to create functional neural circuits again.
Now we know that the dorsal horn is so welcoming in this way, it might give us a way to repair different types of spinal cord injury, and maybe even reconnect neural circuits in injuries where the spinal cord is severed.
That's still a long way off for now, but it's exciting to get such a close look at how surgeons and the human body can work together to repair injuries. We're looking forward to seeing where the research leads next.
The findings have been published in Frontiers in Neurobiology.