Scientists have discovered the neural headquarters for REM sleep – that dreamy brain state where the eyes are the only part of the body actively moving.
When this circuit at the top of the brainstem is triggered in mice, researchers can make animals slip into REM (rapid eye movement) sleep, even if they are wide awake to start with.
If the findings extend to humans, we will be one big step closer to understanding the biology of sleep and why it can go awry.
The knowledge could even help us manipulate REM sleep for the better in humans with sleep apnea, narcolepsy, frequent distressing nightmares, or REM disorders, which cause people to act out their dreams with movement or vocalizations – such as talking in their sleep.
Mysteries around REM sleep abound, and research is made all the more difficult by the fact that scientists still don't know where the control center for REM sleep sits in the brain, or even if there's a control center at all.
For decades now, some researchers have suspected that neurons in the mammal brainstem play a critical role in the onset of REM. If the brainstem is cut out of the picture in cats, for instance, proper REM sleep cannot be generated and the animals begin to act out their dreams.
In humans with known brainstem degeneration, like that seen in Parkinson's disease, REM sleep can be disordered in similar ways.
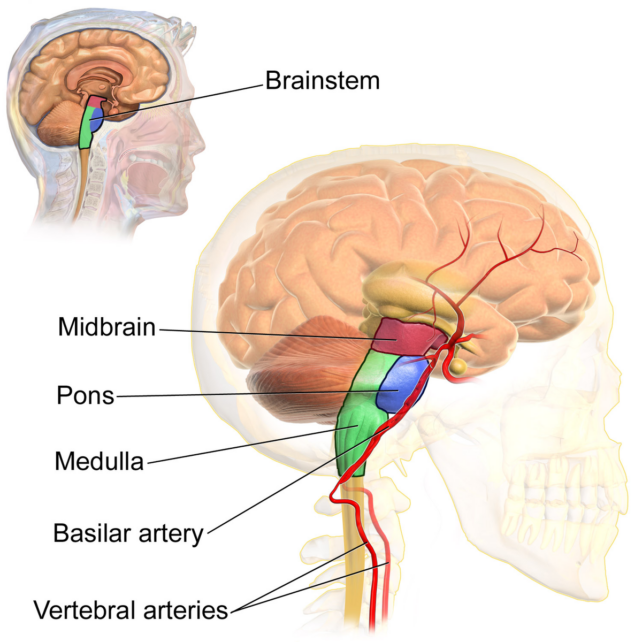
Over the years, further experiments on rodents have found evidence it is the pons, at the top of the brainstem, that is the 'control center' for the usual loss of muscle tension that limits movement during REM sleep.
But because the neurons that promote wakefulness in this part of the brain are intermingled with those that promote sleep, pinpointing the exact pathways responsible for this crucial phase of sleep has proved challenging.
Neuroscientist Mitsuaki Kashiwagi from the University of Tsukuba and the University of Tokyo has now led a team in Japan and France to a cluster of REM-related neurons in the dorsal part of the pons.
In mice, these neurons express a corticotropin-releasing hormone-binding protein, so they are called Crhbp+ neurons.
These cells project from the pons to neurons in the medulla oblongata, the region of the brainstem just underneath. These are called Nos1+ neurons because they express nitric oxide synthase 1. NOs1+ neurons then connect back to the Crhbp+ neurons and on to neurons in the forebrain.
This loop from the pons to the medulla and back again could operate as a core circuit of REM sleep, Kashiwagi and his colleagues argue.
When the team deleted pons neurons from the positive feedback loop, the mice showed reduced sleep and impaired muscle relaxation during REM sleep.
When the pons neurons that extend to the medulla were activated, however, mice slipped into REM sleep faster, and the number and length of REM episodes during their sleep increased at the expense of wakefulness.
In the medulla, Nos1+ neurons strongly promoted REMS, projecting to multiple areas involved in REM activity.
In fact, activating these neurons in mice caused direct transitions from wakefulness to REM sleep. Even when non-REM sleep came first, it was highly shortened, with the mice slipping into REM sleep faster. Neurons that extend to the forebrain seem to inhibit wakefulness.
Humans who suffer from narcolepsy are known to go from wakefulness straight to REM sleep, but otherwise this jump is highly unusual.
"Having established Crhbp as a marker for sleep-regulating neurons, we examined whether these neurons are affected in Parkinson's patients with REMS behavior disorder," the authors explain.
Sure enough, the team found Crhbp-immunoreactive neurons are largely reduced in this cohort, "providing insight into the mechanisms underlying the sleep deficits characterizing this disease."
In a mouse model of Parkinson's disease, the researchers showed activation of Crhbp+ neurons in the pons can reverse the observed sleep abnormalities.
The next step, Kashiwagi and his colleagues say, is to record the activity of these neurons at a single-cell resolution to figure out what they are really doing, and why.
The study was published in Cell.
