For the first time, scientists have been able to make a three-dimensional, real-time recording of the moment a virus hijacks a cell, giving us a deeper level of understanding of how infections take hold in the body.
The microscopic nature film lasts two-and-a-half minutes, and shows a genetically sterile virus many thousands of times smaller than a grain of sand traveling along a wall of human intestinal cells as it looks for an entry point.
Understanding how viruses break into cells is crucial in working out better ways of defending against them, but tracking these particles is incredibly difficult – not least because they are so much smaller than the cells they're navigating.
"It's like you're trying to take a picture of a person standing in front of a skyscraper," says chemist Courtney Johnson, from Duke University in North Carolina. "You can't get the whole skyscraper and see the details of the person in front of it with one picture."
What's more, virus particles move much faster outside the cell than inside it, making it even trickier to come up with an imaging process that is fine-tuned to cope with these varying sizes and speeds.
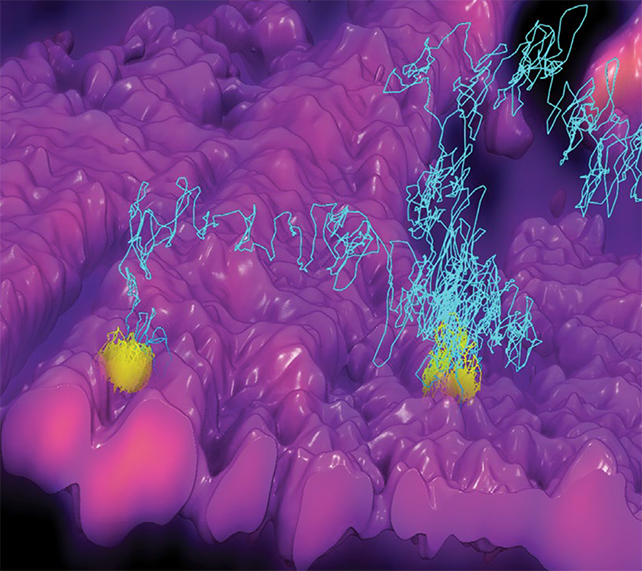
The solution in this case is a system called 3D-TrIm, or 3D Tracking and Imaging Microscopy. It's basically two microscopes in one: the first to 'lock on' to the fast-moving particle, and the second to capture 3D pictures of the surrounding cells. It's a little bit like a satellite navigation app tracking your car's location in the middle of a wider landscape.
With the virus particle illuminated via a special fluorescent label, it's position can be plotted 1,000 times a second, giving researchers a look at its movements across a key period in the infection process in unprecedented detail.
In the Duke University video below, the meandering path of the virus can be seen as a squiggly purple line.
"Sometimes when I present this work people ask, 'is this a video game or a simulation?'" says Johnson. "No, this is something that came from a real microscope."
We're all breathing in millions of viruses every day, the vast majority of which fail to do any harm – but scientists want to learn more about how certain viruses break through the protective layer of cells and mucus covering the airways and gut to establish an infection.
This new 3D-Trim method should help, though it has its limitations: virus particles need to be labeled prior to imaging so they can be seen, and the fluorescent dye on them needs to be engineered to last long enough to enable researchers to track the whole infection process.
However, the team behind 3D-Trim says the potential is there for the system to get better quickly, and to be adapted to other types of medical diagnostics – whether that's keeping watch over viruses or monitoring drug delivery.
"Importantly, the application of this technique can be extended to any system where fast dynamics of nanoscale objects occur over large volumetric scales, including delivery of nanoscale drug candidates to the lungs and through leaky tumor vasculature," write the researchers in their published paper.
The research has been published in Nature Methods.