Health care providers and patients have traditionally thought that infections patients get while in the hospital are caused by superbugs they're exposed to while they're in a medical facility.
Genetic data from the bacteria causing these infections – think CSI for E. coli – tells another story: Most health care-associated infections are caused by previously harmless bacteria that patients already had on their bodies before they even entered the hospital.
Research comparing bacteria in the microbiome – those colonizing our noses, skin and other areas of the body – with the bacteria that cause pneumonia, diarrhea, bloodstream infections and surgical site infections shows that the bacteria living innocuously on our own bodies when we're healthy are most often responsible for these bad infections when we're sick.
Our newly published research in Science Translational Medicine adds to the growing number of studies supporting this idea. We show that many surgical site infections after spinal surgery are caused by microbes that are already on the patient's skin.
Surgical infections are a persistent problem
Among the different types of heath care-associated infections, surgical site infections stand out as particularly problematic. A 2013 study found that surgical site infections contribute the most to the annual costs of hospital-acquired infections, totaling over 33 percent of the US$9.8 billion spent annually.
Surgical site infections are also a significant cause of hospital readmission and death after surgery.
In our work as clinicians at Harborview Medical Center at the University of Washington – yes, the one in Seattle that "Grey's Anatomy" was supposedly based on – we've seen how hospitals go to extraordinary lengths to prevent these infections. These include sterilizing all surgical equipment, using ultraviolet light to clean the operating room, following strict protocols for surgical attire and monitoring airflow within the operating room.
Still, surgical site infections occur following about 1 in 30 procedures, typically with no explanation. While rates of many other medical complications have shown steady improvement over time, data from the Agency for Healthcare Research and Quality and the Centers for Disease Control and Prevention show that the problem of surgical site infection is not getting better.
In fact, because administering antibiotics during surgery is a cornerstone of infection prevention, the global rise of antibiotic resistance is forecast to increase infection rates following surgery.
BYOB (Bring your own bacteria)
As a team of physician-scientists with expertise including critical care, infectious diseases, laboratory medicine, microbiology, pharmacy, orthopedics and neurosurgery, we wanted to better understand how and why surgical infections were occurring in our patients despite following recommended protocols to prevent them.
Prior studies on surgical site infection have been limited to a single species of bacteria and used older genetic analysis methods. But new technologies have opened the door to studying all types of bacteria and testing their antibiotic resistance genes simultaneously.
We focused on infections in spinal surgery for a few reasons. First, similar numbers of women and men undergo spine surgery for various reasons across their life spans, meaning our results would be applicable to a larger group of people.
Second, more health care resources are expended on spinal surgery than any other type of surgical procedure in the US Third, infection following spine surgery can be particularly devastating for patients because it often requires repeat surgeries and long courses of antibiotics for a chance at a cure.
Over a one-year period, we sampled the bacteria living in the nose, skin and stool of over 200 patients before surgery. We then followed this group for 90 days to compare those samples with any infections that later occurred.
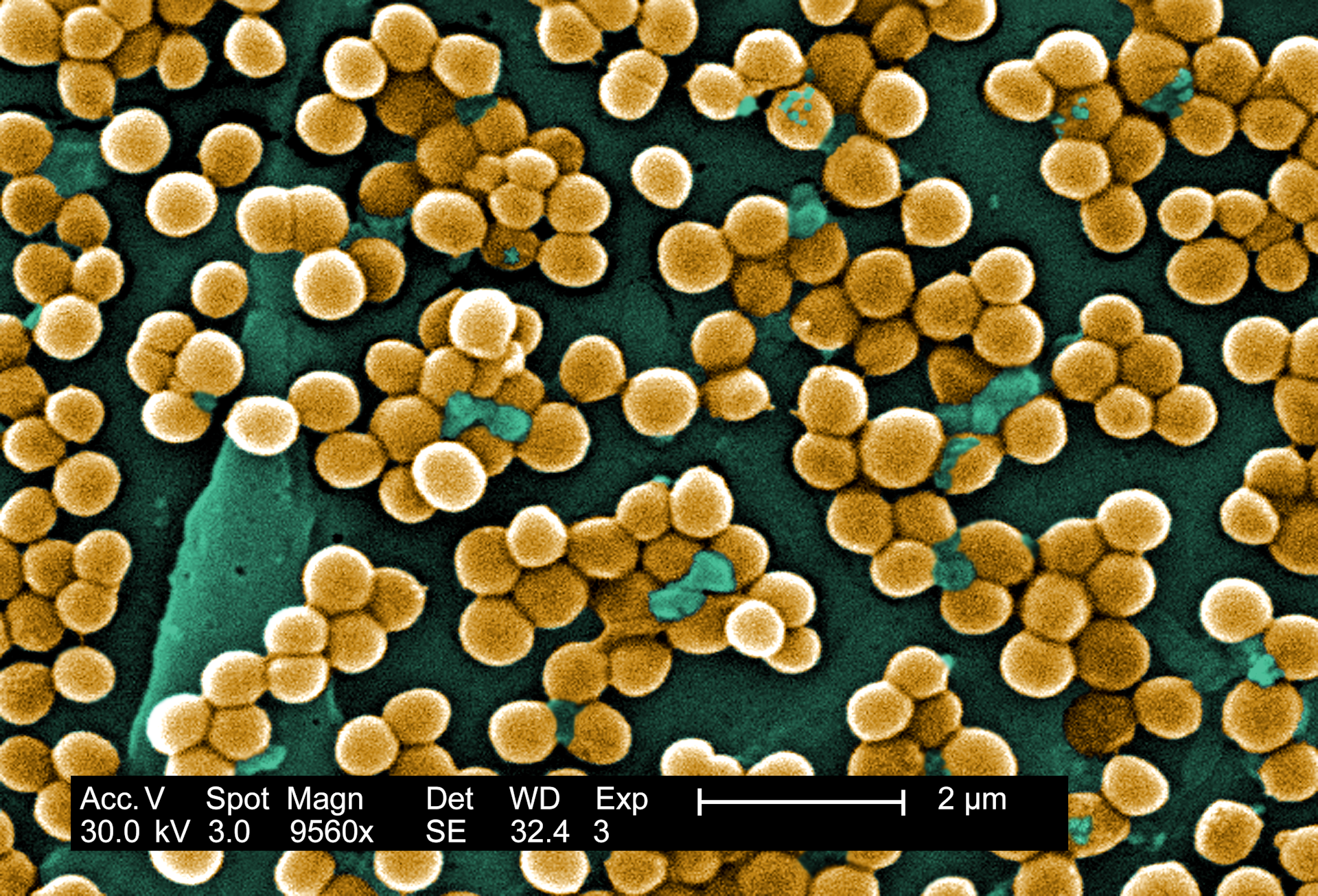
Our results revealed that while the species of bacteria living on the back skin of patients vary remarkably between people, there are some clear patterns. Bacteria colonizing the upper back around the neck and shoulders are more similar to those in the nose; those normally present on the lower back are more similar to those in the gut and stool. The relative frequency of their presence in these skin regions closely mirrors how often they show up in infections after surgery on those same specific regions of the spine.
In fact, 86 percent of the bacteria causing infections after spine surgery were genetically matched to bacteria a patient carried before surgery. That number is remarkably close to estimates from earlier studies using older genetic techniques focused on Staphylococcus aureus.
Nearly 60 percent of infections were also resistant to the preventive antibiotic administered during surgery, the antiseptic used to clean the skin before incision or both.
It turns out the source of this antibiotic resistance was also not acquired in the hospital but from microbes the patient had already been living with unknowingly. They likely acquired these antibiotic-resistant microbes through prior antibiotic exposure, consumer products or routine community contact.
Preventing surgical infections
At face value, our results may seem intuitive – surgical wound infections come from bacteria that hang out around that part of the body. But this realization has some potentially powerful implications for prevention and care.
If the most likely source of surgical infection – the patient's microbiome – is known in advance, this presents medical teams with an opportunity to protect against it prior to a scheduled procedure. Current protocols for infection prevention, such as antibiotics or topical antiseptics, follow a one-size-fits-all model – for example, the antibiotic cefazolin is used for any patient undergoing most procedures – but personalization could make them more effective.
If you were having a major surgery today, no one would know whether the site where your incision will be made was colonized with bacteria resistant to the standard antibiotic regimen for that procedure. In the future, clinicians could use information about your microbiome to select more targeted antimicrobials. But more research is needed on how to interpret that information and understand whether such an approach would ultimately lead to better outcomes.
Today, practice guidelines, commercial product development, hospital protocols and accreditation related to infection prevention are often focused on sterility of the physical environment. The fact that most infections don't actually start with sources in the hospital is probably a testament to the efficacy of these protocols.
But we believe that shifting toward more patient-centered, individualized approaches to infection prevention has the potential to benefit hospitals and patients alike.![]()
Dustin Long, Assistant Professor of Anesthesiology, School of Medicine, University of Washington and Chloe Bryson-Cahn, Associate Professor of Allergy and Infectious Diseases, School of Medicine, University of Washington
This article is republished from The Conversation under a Creative Commons license. Read the original article.