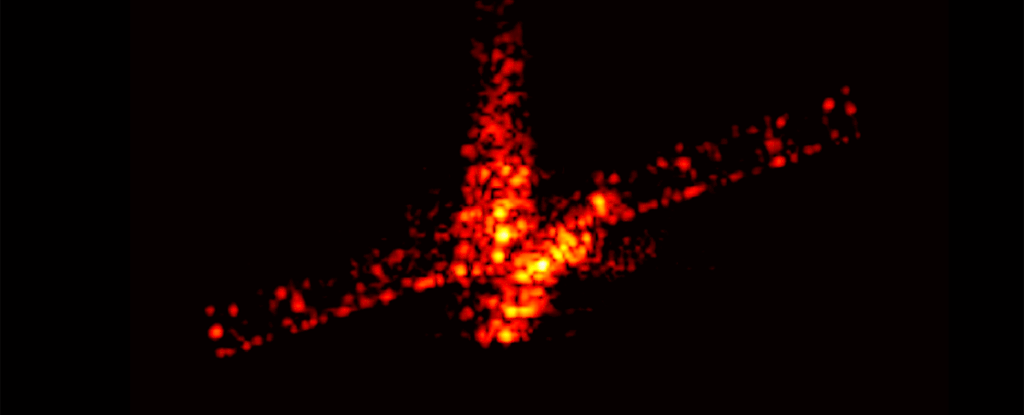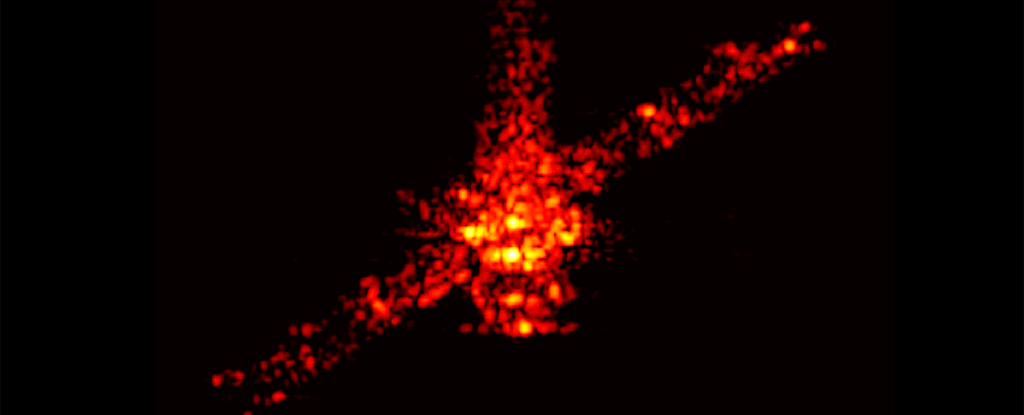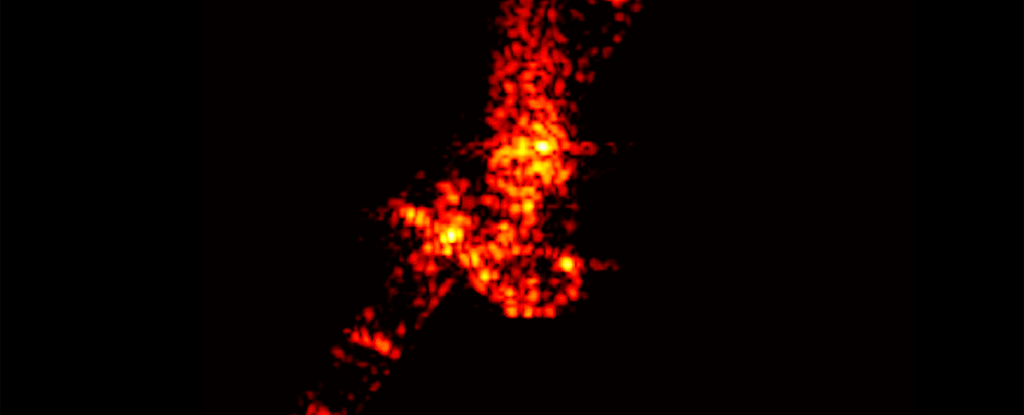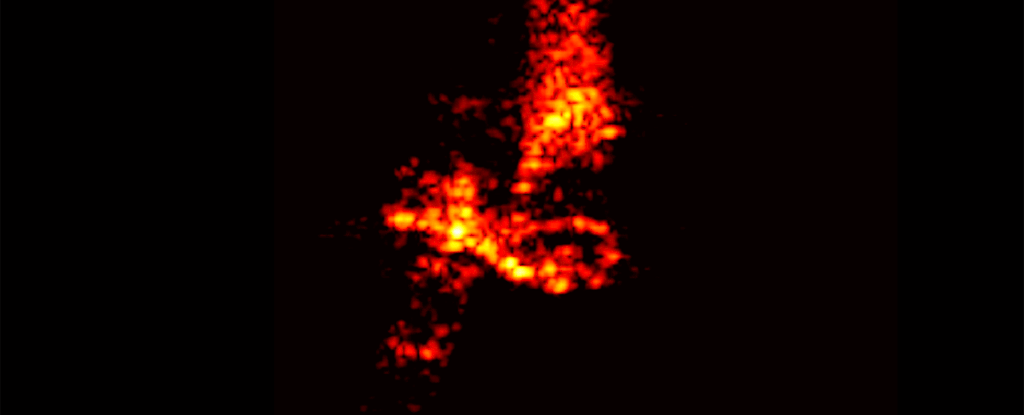
Even the space junk designed with disposal in mind is a polluting presence around Earth, a new study has found. Those chunks of rocket and space station and dead satellites that burn up on atmospheric reentry leave miniscule traces of metal lingering in our planet's atmosphere.
At this time, it's not known what the impact of those traces might be. But with the growing rate of launching things into space, the amount of metal vapor in the stratosphere is only predicted to increase.
It's a discovery, says a team of researchers led by physicist Daniel Murphy of the National Oceanic and Atmospheric Administration (NOAA) that calls for the investigation of the effects of metal vapor in the atmosphere, and a projection of how that will change over time.
"At present, the refractory material in stratospheric particles is mostly iron, silicon, and magnesium from the natural meteoric source," the researchers write in their new paper.
"However, the amount of material from the reentry of upper-stage rockets and satellites is projected to increase dramatically in the next 10 to 30 years. As a result, the amount of aluminum in stratospheric sulfuric acid particles is expected to become comparable to or even exceed the amount of meteoric iron, with unknown consequences for inclusions and ice nucleation."

Although there's a lot of junk in Earth orbit from the early years of the human space age, more recent launches have been made with a limited life span in mind. Spacecraft that will eventually deorbit and fall back down to Earth are being designed, using materials that will burn up in the upper atmosphere, rather than come crashing down to the surface.
But it's unclear what happens to the vaporized byproducts of reentry. Murphy and his colleagues wanted to find out whether vapor from these deorbits lingered in the stratosphere. They took samples of the stratospheric aerosols and analyzed them using the Particle Analysis by Laser Mass Spectrometer (PALMS) instrument aboard NASA's WB-57 high altitude aircraft.
Aerosols in the stratosphere are mostly droplets of sulfuric acid, produced by the oxidation of the carbonyl sulfide gas that occurs both naturally and as a pollutant in the atmosphere. These droplets can contain traces of metals and silicon obtained from the atmospheric entry of meteors, whose surfaces vaporize as they fall.
The team analyzed some 500,000 individual aerosol droplets, looking for traces of metals used in spacecraft manufacture. They detected around 20 metals.
Some of those metals were in ratios consistent with vaporizing meteors: but others, like lithium, aluminum, copper, and lead, exceeded the expected amounts from meteor ablation. The excess, the team found, was consistent with ratios expected from spacecraft manufacture.
Other metals they found, such as niobium and hafnium, are common in spacecraft, but not common at all in meteors. Overall, the team found some 10 percent of stratospheric aerosols above a certain size retained particles of vaporized spacecraft.
There are several effects this could have on Earth and the atmosphere. The presence of these particles could affect how water freezes into ice in the stratosphere, and influence the size of stratospheric aerosol particles. They could also induce salt deposition on aerosol particles, and alter the stratospheric refraction of light.
These may seem like subtle changes, but they could have unintended consequences that we really ought to investigate, the researchers say.
"The space industry has entered an era of rapid growth. With tens of thousands of small satellites planned for low earth orbit, that increased mass will be divided into many more reentry events," they conclude.
"Given that 10 percent of stratospheric particles now contain enhanced aluminum, with many more reentry events, it is likely that in the next few decades, the percentage of stratospheric sulfuric acid particles that contain aluminum and other metals from satellite reentry will be comparable to the roughly 50 percent that now contain meteoric metals."
The research has been published in the Proceedings of the National Academy of Sciences.