A sequence of stress signals among specialized clean-up cells in the brain could at last reveal why some immune responses can cause significant nerve degeneration that results in the loss of memory, judgement, and awareness behind Alzheimer's disease.
Blocking this pathway in mouse brains modeled on Alzheimer's prevented damage to their synapse connections and reduced the buildup of potentially toxic tau proteins – both hallmarks of the condition.
The researchers, led by a team from the City University of New York (CUNY), believe this pathway – called the integrated stress response (ISR) – causes brain immune cells called microglia to go 'dark' and start damaging rather than benefiting the brain.
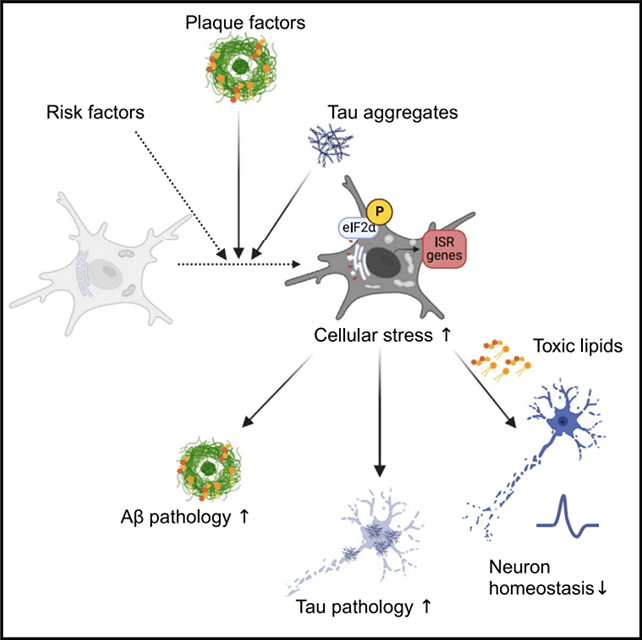
"We set out to answer what are the harmful microglia in Alzheimer's disease and how can we therapeutically target them," says CUNY neuroscientist Pinar Ayata.
"We pinpointed a novel neurodegenerative microglia phenotype in Alzheimer's disease characterized by a stress-related signaling pathway."
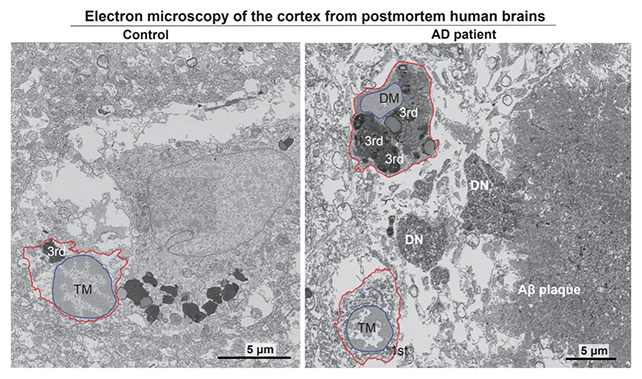
Haywire immune cells have previously been linked to Alzheimer's, prompting the team to use an electron scanning process to identify the buildup of dark microglia in human brains affected by Alzheimer's.
Finding around twice as many stressed microglia in brains with the condition compared with healthy brains, the researchers went on to show how the ISR pathway was causing dark microglia to release harmful lipids into the brain's tissues.
It was these damaging fats that caused the damage to synapses and neuron communication seen in Alzheimer's.
As is often the case with Alzheimer's research, a better understanding of how the disease operates can also give scientists more ideas for how to treat it. If treatments that block ISR can work safely and effectively in humans, the method could potentially slow the chaos that Alzheimer's causes in our own brain.
"These findings reveal a critical link between cellular stress and the neurotoxic effects of microglia in Alzheimer's disease," says molecular biologist Anna Flury from CUNY.
"Targeting this pathway may open up new avenues for treatment by either halting the toxic lipid production or preventing the activation of harmful microglial phenotypes."
The team behind this study found that the misfolded protein malfunctions that often drive dementia could be triggering the ISR to begin with, meaning that these signals are both a result of Alzheimer's and a reason for its further progression.
Further studies should make this relationship clearer, now that we have a better idea of how the ISR pathway and dark microglia act in the brain – and from there, hopefully, new approaches to therapies.
"Such treatments could significantly slow or even reverse the progression of Alzheimer's disease, offering hope to millions of patients and their families," says neuroscientist Leen Aljayousi, from CUNY.
The research has been published in Neuron.