Alzheimer's disease is the most common cause of dementia; if we are to alleviate its impact, we need to understand as much as possible about how it gets started and the effects it has on brain cells – and a new study reveals that the development of the disease can cause cells to seriously overheat.
We know that Alzheimer's is characterized by the proteins amyloid-beta and tau clumping together in the brain, killing off cells and causing the brain to shrink. The new study shows how amyloid-beta can destroy a cell "like frying an egg" by raising its temperature.
One of the most challenging aspects of researching Alzheimer's is the fact we don't fully understand what causes amyloid-beta to build up in the brain; however, in this latest study, researchers were able to observe it triggering this temperature change, known technically as intracellular thermogenesis.
"Once the aggregates have formed, they can exit the cell and be taken up by neighboring cells, infecting healthy amyloid-beta in those cells," says chemical engineer Chyi Wei Chung, from the University of Cambridge in the UK.
Alzheimer's is a notoriously difficult disease to investigate, because it takes a long time to develop; its full impact on someone's brain can only be examined in detail by analyzing brain tissue after someone has died.
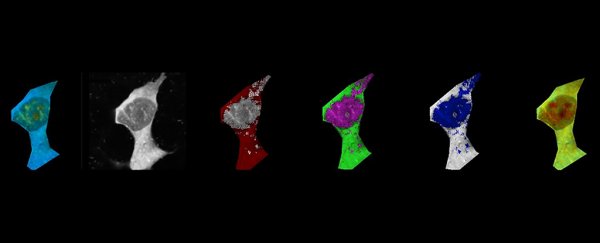
This particular study was made possible through tiny temperature sensors called fluorescent polymeric thermometers (FPTs).
The team added amyloid-beta to human cell lines in the lab to trigger the aggregation process. Once the amyloid-beta protein began to form into threads known as fibrils, the average temperature of the cells started to rise, compared with cells that hadn't had any of the protein artificially added. That then leads to a chain reaction.
"As the fibrils start elongating, they release energy in the form of heat," says neuroscientist Kaminski Schierle, from the University of Cambridge.
"Amyloid-beta aggregation requires quite a lot of energy to get going, but once the aggregation process starts, it speeds up and releases more heat, allowing more aggregates to form."
By inhibiting amyloid-beta aggregation in other cells, the researchers were able to isolate and identify fibril formation as the cause of thermogenesis. It's possible that future Alzheimer's treatments could look at preventing aggregation as a way of keeping brain cells cool and – crucially – alive.
"We show, for the first time in live cells, that Aβ42 [amyloid-beta subvariant 42] elongation is directly responsible for elevating cell-averaged temperatures," the team writes.
In addition to the laboratory experiments, computer models were used to assess how amyloid-beta could work within a cell to increase temperatures, potentially bringing us closer to a full understanding of the disease that affects tens of millions of lives.
Any resulting treatments are still a long way off, but the study clears up one of the mysteries around the disease. It had been thought that damage to the cell batteries or mitochondria might have been leading to thermogenesis instead.
"Thermogenesis has been associated with cellular stress, which may promote further aggregation," says Chung.
"We believe that when there's an imbalance in cells, like when the amyloid-beta concentration is slightly too high and it starts to accumulate, cellular temperatures increase."
The research has been published in the Journal of the American Chemical Society.