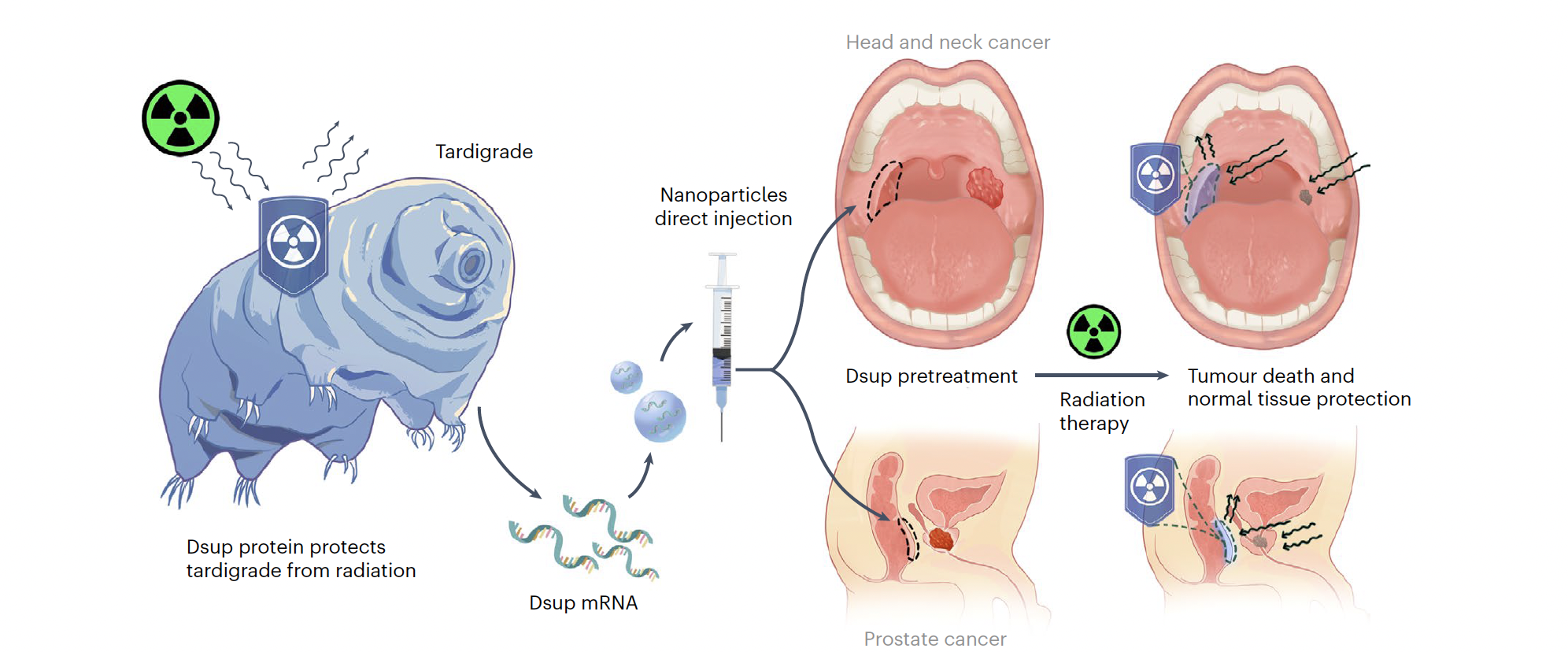
When it comes to surviving radiation, tardigrades really know their stuff, shrugging off doses that would annihilate most other life forms. Now researchers are using this knowledge to find ways to protect healthy cells during cancer treatments.
A team led by Ameya Kirtane from Harvard Medical School and Jianling Bi from the University of Iowa has isolated this superpower in the form of messenger RNA, which when injected into cells protects them from radiation.
When people undergo radiotherapy for cancer, it's not just the tumor that suffers. The radiation causes DNA breaks in healthy cells, too, leading to massive cell death and inflammation, which is responsible for the treatment's unpleasant side-effects.
"It can manifest as something as simple as mouth sores, which can limit a person's ability to eat because it's so painful, to requiring hospitalization because people are suffering so terribly from the pain, weight loss, or bleeding," University of Iowa radiation oncologist James Byrne says.
Despite their cute monikers like moss piglet and water bear, the microscopic, eight-legged animals known as tardigrades are notoriously tough. Aside from surviving the hottest setting of your oven and pressures of 7.5 Gpa, they can handle around a thousand times the dose of ionizing radiation that would kill a human.
They can do this because of their ability to produce a unique protein Dsup (short for 'damage suppressing'), which helps them tolerate both the initial blast and the hydroxyl radicals that form in cells as a result, which would otherwise tear up one or even both strands of DNA.
Scientists have had their eyes on this protein as a potential aid to cancer treatment since it was discovered in 2016, and now they're one step closer.

That 2016 study showed that when expressed in human cells, Dsup reduces X-ray-induced DNA damage by about 40 percent, which is why the researchers are hopeful it could protect cancer patients from the serious side-effects of their treatment.
But Dsup has to be inside a cell's nucleus to work. Delivering this protein directly into each cell is not feasible, and integrating the genes for Dsup directly into DNA has its own risks.
"One of the strengths of our approach is that we are using a messenger RNA, which just temporarily expresses the protein, so it's considered far safer than something like DNA, which may be incorporated into the cells' genome," Kirtane says.
By wrapping the mRNA in specific polymer-lipid nanoparticles (one design best-suited to the colon, and one ideal for the mouth) they were able to smuggle the strands into lab-grown cells where they were used to generate large amounts of Dsup before disintegrating.
"We thought that perhaps by combining these two systems – polymers and lipids – we may be able to get the best of both worlds and get highly potent RNA delivery. And that's essentially what we saw," Kirtane says.
Importantly, delivering the Dsup in mRNA 'recipe' format also prevents the protective tardigrade armor from sneaking their way into cells the radiation is supposed to kill, such as those making up the tumor.
To make sure this works in action, the team injected Dsup-encoding mRNA into mice who, 6 hours later, received a dose of radiation roughly equivalent to one that might be administered to a human cancer patient.
One group of mice received mRNA treatment and radiation to the mouth; the other, to the rectum. And some extra mice were given the radiation without the protection of Dsup, to provide a baseline for comparison.
The 'rectal' group experienced about half as many radiation-induced double-stranded DNA breaks, compared with controls that did not receive Dsup protection. The 'mouth' group had about one third of the breaks of their peers. And the mRNA treatment seemed to have no effect on tumor volume.
This research is just the beginning: the sample sizes were very small, and of course, we can't predict how human bodies will react to a treatment based solely on tests on lab-grown cells or mice. But it's enough to prompt further investigation, especially now they know they can get the mRNA safely into a cell without conferring the benefit on the cancer.
"The use of Dsup mRNA delivery may be co-opted for several other clinical applications, including protection of normal tissue from DNA-damaging chemotherapies or progressive degeneration of specific tissues, cancer predisposition, chromosomal instability and hypersensitivity to DNA-damaging agents," the authors write.
"In addition to cancer-related applications, the use of Dsup protein can be extended to total body exposure to space radiation or as prophylaxis against nuclear radiation exposure."
This research was published in Nature Biomedical Engineering.
