As analytical methods get more sophisticated, existing scientific models are constantly reexamined. The latest to come under scrutiny is the way molecules are organized at the surface of a volume of salt water.
Researchers from the University of Cambridge in the UK, and the Max Planck Institute for Polymer Research in Germany, found that electrically charged particles, or ions, aren't active on the very surface of the solution, as was previously thought – instead, they're located in a subsurface layer.
The discovery will require textbook models to be re-drawn, a University of Cambridge press release explains.
"Our work demonstrates that the surface of simple electrolyte solutions has a different ion distribution than previously thought and that the ion-enriched subsurface determines how the interface is organized," says theoretical chemist Yair Litman from the University of Cambridge.
To make their discovery, the team used an upgraded version of a laser radiation technique called vibrational sum-frequency generation (VSFG), which measures molecular vibrations at the smallest scales with impressive accuracy.
Together with models powered by neural networks, this improved technique meant that the researchers were able to see whether ions at the surface were positively charged (cations) or negatively charged (anions).
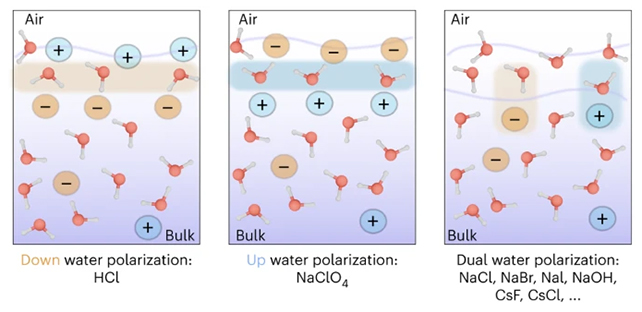
As well as detecting the subsurface layer of ions, the new study also reveals that these ions can be oriented in both up and down directions – referring to the actual physical arrangement of the molecules – rather than just one direction.
"At the very top there are a few layers of pure water, then an ion-rich layer, then finally the bulk salt solution," says Litman.
In simple terms the experiment reveals what's going on at the very borders of most simple liquid electrolyte solutions. The molecular arrangement informs how they will react with what's around them.
A thorough understanding of these layers and their arrangement can inform all kinds of other models – such as those we have for the surface of the ocean, for example, which are vital for projecting the effects of climate change on the atmosphere.
And as well as enhancing our understanding of the world around us, the researchers suggest that their work could also help in the development of any kind of technology where solids and liquids have to be combined – including batteries.
"These types of interfaces occur everywhere on the planet, so studying them not only helps our fundamental understanding but can also lead to better devices and technologies," says molecular physicist Mischa Bonn from the Max Planck Institute for Polymer Research.
"We are applying these same methods to study solid/liquid interfaces, which could have potential applications in batteries and energy storage."
The research has been published in Nature Chemistry.
