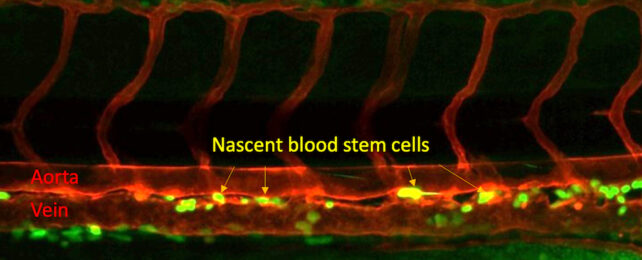
The development of blood stem cells relies on a seemingly unrelated microbe-sensing protein receptor, according to a new study.
The discovery could break new ground in the ongoing quest to produce blood stem cells from a person's own blood – thereby negating the need for bone marrow transplants.
The protein receptor in question, called Nod1, is already known for its role in helping recognize bacterial infections in the body and rallying an immune response, the study's authors note.
But according to their research, Nod1 also seems to serve a different purpose much earlier in life, when an embryo's vascular system is still developing.
Led by Raquel Espin Palazon, a geneticist at Iowa State University, the study suggests this microbial sensor helps embryos force some of their vascular endothelial cells to become blood stem cells.
That could be valuable information, given its potential for shedding light on how an embryo makes blood stem cells – and perhaps how we can grow them much later in life, too.
"This would eliminate the challenging task of finding compatible bone marrow transplant donors and the complications that occur after receiving a transplant, improving the lives of many leukemia, lymphoma, and anemia patients," Espin Palazon says.
Blood stem cells are progenitors of all white and red cells in our blood, producing all the components of our blood in a process called hematopoiesis.
These blood stem cells, also known as hematopoietic stem cells, arise themselves in the body before birth, developing from endothelial cells within an embryo's aorta.
Yet while that much was already clear, there have been few details about what triggers this important process in an embryo.
"We know blood stem cells form from endothelial cells, but the factors that set up the cell to switch identity were enigmatic," Espin Palazon says. "We didn't know that this receptor was needed or that it was needed this early, before blood stem cells even form."
The researchers first homed in on Nod1 by analyzing public databases of human embryos, then studied the receptor further using zebrafish, a commonly used model organism that shares roughly 70 percent of its genome with humans.
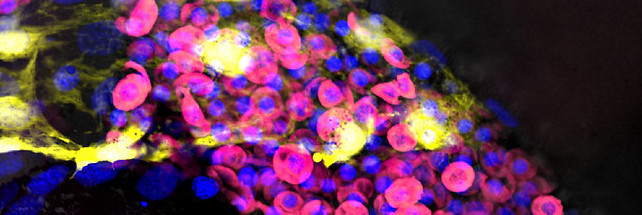
By inhibiting or boosting Nod1, the researchers demonstrated a positive correlation with the creation of blood stem cells.
To shed more light on Nod1 and blood development in humans, the study's authors also collaborated with the Children's Hospital of Philadelphia, where researchers produce human-induced pluripotent stem cells.
Although these are generated from adult body cells, researchers genetically reprogram them to mimic the pluripotent stem cells – those capable of producing many different cell types – found in embryos.
Induced pluripotent stem cells can generate most types of blood cells, but they can't create functional blood stem cells. Still, inhibiting Nod1 caused these induced pluripotent stem cells to produce less blood, mirroring the effect seen in the zebrafish's blood stem cells.
Most of a person's blood stem cells reside in their bone marrow, so patients with certain blood disorders often need a bone marrow transplant to provide a vital supply of blood stem cells.
But armed with this evidence about Nod1's role in creating blood stem cells in embryos, scientists have new hope for devising a way to produce new blood stem cells from human samples, potentially even from patients' own blood.
That could help avoid not only the logistical challenges of arranging and performing bone marrow transplants, the researchers note, but also complications like graft-versus-host disease, in which transplanted immune cells recognize the host as foreign and attack the recipient's cells.
"This would be a huge advancement for regenerative medicine," Espin Palazon says.
More research is still needed not only to understand how exactly the body creates blood stem cells, the researchers say, but also when each step needs to occur.
"The timing is so crucial. It's like when you're cooking and you need to add ingredients in a specific order," Espin Palazon says.
"My group at Iowa State University will continue working toward a life without blood disorders," she adds. "I believe our investigations will pave the road to finally create therapeutic-grade blood stem cells to cure blood disorder patients."
The study was published in Nature Communications.