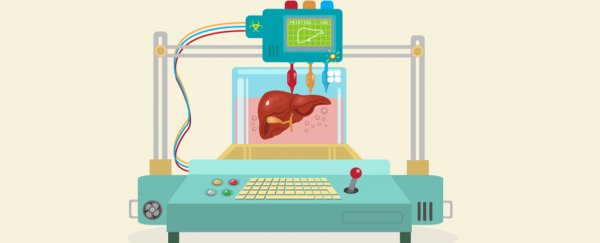
Body organs such as kidneys, livers and hearts are incredibly complex tissues. Each is made up of many different cell types, plus other components that give the organs their structure and allow them to function as we need them to.
For 3D printed organs to work, they must mimic what happens naturally – both in terms of arrangement and serving a biological need. For example, a kidney must process and excrete waste in the form of urine.
Our latest paper shows a new technique for 3D printing of cells and other biological materials as part of a single production process. It's another step towards being able to print complex, living structures.
But it's not organ transplants we see as the most important possible consequence of this work.
There is already evidence that 3D cell printing is a technology useful in drug development, something that may reduce the burden on animals for testing and bring new treatments to market more quickly and safely.
How we 3D bioprint
3D printing was first developed for rapid fabrication of industrial parts using methods known as sterolithography and fuse deposition modelling.
Add "biology" (that is, cells) to the printing technique and it becomes an entirely new process: 3D bioprinting.
3D bioprinting requires sterile conditions to avoid contamination of the bioprinted sample, and an appropriate temperature and humidity so the cells don't die. Also, the plastic materials traditionally used in 3D printing cannot be used in bioprinting, as they require high temperatures or toxic solvents.
We and other researchers around the world are developing materials that can be manipulated in a 3D printer while causing minimal harm to the cells.
However, each cell type that makes up the different tissues of the human anatomy requires a unique mechanical environment. Each requires unique structural supports to function normally.
As an example, bones are a resistant and brittle material, muscles of the heart are elastic, tough materials, and internal organs such as the liver are soft and compressible.
In a recent publication, we and our colleagues show that new materials extracted from marine algae can be used to 3D bioprint human stem cells in distinct environments, and without harming the cells. We believe that these findings pave the way toward the printing of complex tissue structures.
 Steffen Harr
Steffen Harr
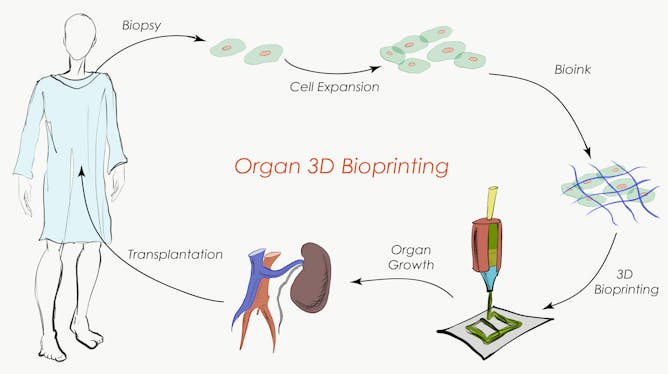
Hoping for new organs
Currently, patients needing replacement organs must wait for availability (from living or deceased donors) and are then required to be on immunosuppressive drugs for most of the rest of their lives, causing side effects and creating a tremendous cost for the healthcare system.
The development of 3D-printed biological tissues for organ replacement hopes to offer a new solution for the 1,500 patients on the organ receiver waiting list every year in Australia.
But printing of entire organs is an incredibly complex process, one that takes weeks of time that a patient may not have up his or her sleeve.
Also, while this process is somewhat advanced for relatively simple tissues such as skin, the next phase of the technology requires incorporation of nerves, blood vessels and lymphatic vessels that would integrate with the host system to create transplantable whole organs such as kidneys, lungs, hearts or livers.
 Steffen Harr
Steffen Harr
We're probably many many years and millions of dollars away from being able to bioprint whole, functional human organs.
But there's another way bioprinted cells can be used: for testing new drugs in the laboratory.
Bioprinted cells for drug testing
Using current methods, bringing a new drug to market has been estimated to cost US$2.5 billion, and can take more than ten years from start to finish.
Even if you manage to identify a new candidate drug, the likelihood of regulatory approval is low: in 2016, less than 10 percent were approved.
When starting human clinical trials, the probability of a drug to make it to the market is between 10 and 15 percent depending on the type of molecule , with illness or even death for participants.
We know that these drugs mainly fail due to poor efficacy in humans despite promising results in animals. This disconnect is due to the different physiology between species: rodents and other trial animals are very different from humans in many key ways.
3D printing technology allows us to print more complex 3D models that reproduce aspects of the liver, kidneys or heart muscles that are suitable to test and identify novel pharmaceutical molecules. These models are already starting to be used by multinational pharmaceutical companies.
While the use of animals in research is still inevitable, the regulatory agency the Food and Drug Administration and its new director have already started to consider integrating alternatives for drug safety and efficacy assessment.
The idea that bioprinted tissues have promise for drug development is already recognised, with funding agencies here in Australia and globally supporting projects.
 Steffen Harr
Steffen Harr
Toward the end of the animal testing?
In 2013 the European Union passed a new law prohibiting the use of animal testing for cosmetic development on its territory, and of retailing products tested abroad on animals.
This regulation has accelerated the development of human-based 3D models of skin for the testing of new cosmetic formulations. These resolutions were accepted because the technology was available and has enabled a reduction in the number of research animals.
This is about to be translated in Australia as well.
![]() The changes operated in other industries combined with the exciting technological advances let us have a glance at how 3D bioprinting may be able to contribute to faster and cheaper ways to create effective new drugs.
The changes operated in other industries combined with the exciting technological advances let us have a glance at how 3D bioprinting may be able to contribute to faster and cheaper ways to create effective new drugs.
Aurelien Forget, Associate Lecturer in Macromolecular Chemistry, Queensland University of Technology and Tim Dargaville, ARC Future Fellow, A/Prof Polymer Chemistry, Queensland University of Technology
This article was originally published by The Conversation. Read the original article.
Queensland University of Technology is a sponsor of ScienceAlert. Find out more about their research.