An oral medication approved to treat multiple sclerosis (MS) could also put the brakes on inflammation thought to play a role in the development of Alzheimer's disease, new research suggests.
Successfully passing rounds of expensive and time-consuming clinical trials, the pharmaceutical ponesimod was given the green light to treat MS by the US Food and Drug Administration and European Medicines Agency in 2021.
Now experiments conducted by researchers from the University of Kentucky have produced evidence of the drug blocking overzealous immune activity in the nervous system, which is considered to play a critical role in the onset of dementia.
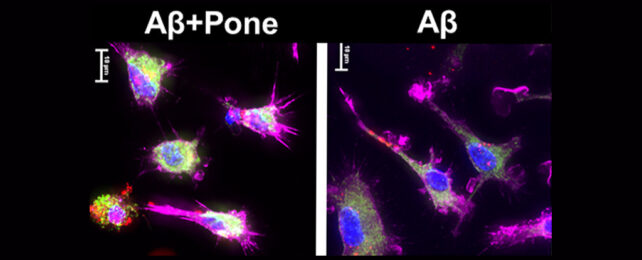
"We are the first to show that ponesimod is effective in a mouse model for Alzheimer's disease," says neuroscientist Erhard Bieberich, the principal investigator on the study.
Similar to another existing MS medication called fingolimod, ponesimod blocks receptors on the surfaces of T and B white blood cells, preventing them from leaving the body's thymus and lymph nodes.
Typically a type of fat called a sphingolipid activates white cells, unlocking their ability to slip into the blood stream and go on the prowl for harmful materials to destroy.
Under 'home detention', these key players in immune responses can no longer rush forth to protect the body from invaders in significant numbers. But they also can no longer antagonize the body's own vital systems, as occurs in organ transplants and autoimmune disorders like lupus, inflammatory bowel disease, and MS.
Immune functions may also play a role in promoting, if not causing Alzheimer's disease.
It's the job of brain-prowling white cells called microglia to clear toxic clumps of beta amyloid and tangles of tau proteins in brain cells.
For some reason, microglia can also switch into overdrive, prompting them to flood their surroundings with signaling chemicals called cytokines that puts the tissues into a state of high alert.
As with MS, this boost of inflammation can damage nerves, leading to the steady degeneration we associate with the symptoms of dementia, such as confusion, loss of judgment, and memory loss.
Since the same sphingolipid that gives T and B cells a hall pass also activates receptors on the microglia, Bieberich and his team wondered if a drug like ponesimod might flick microglia's switches, forcing them to silently focus their efforts on clean-up.
Using mice genetically engineered to present human-like features of Alzheimer's disease, and post-mortem samples of brain tissue donated by Alzheimer's patients, the researchers investigated both the role of the sphingolipid in mediating microglia activity and the consequences of blocking it.
So far the results look encouraging, with clear signs that ponesimod reduces pro-inflammatory cytokines and triggers anti-inflammatory signaling that encourages microglia to gobble up troublesome protein clumps and tangles in the brain.
"In our study, we reprogrammed microglia into neuron-protective cells that clean up toxic proteins in the brain, reduce Alzheimer's neuroinflammatory pathology, and improve memory in the mouse model," says the study's lead author, Zhihui Zhu.
As with any medication that shows promise, there is still a need to replicate the effects safely in people before we can consider this a suitable treatment for human patients. Yet piggy-backing on a pharmaceutical that has proven its safety and efficacy clears a significant amount of red tape and expense.
Having cost-effective ways to slow or even rewind the debilitating symptoms of Alzheimer's disease is quickly becoming a global priority.
More than 55 million people worldwide live with dementia. By the middle of the century, this figure is expected to soar as populations age, nearly tripling to just under 140 million. Most of this growth is expected to take place in developing nations.
"Since this drug is already in clinical use for therapy of relapsing multiple sclerosis, it is immediately available to be used in Alzheimer's disease therapy as well," says Bieberich.
This research was published in eBioMedicine.