It sounds a little bit like the premise of a horror movie, but scientists have determined that the pathogenic fungus Cryptococcus neoformans actually changes in size once it enters a body, increasing its chance of infection.
Out in the wild, the fungus can be found in a variety of different habitats, such as rotting wood or in bird droppings. It shows the same versatility inside the body once inhaled, traveling from the lungs through the bloodstream to other organs.
Once settled inside the human body the pathogen can be responsible for a range of health issues, including the rare though potentially fatal brain swelling condition fungal meningitis.
New findings based on a study in mice could eventually help us treat it more effectively.
"Cryptococcus cells in the lungs are very diverse with different sizes and different appearances," says pathologist Jessica Brown, from University of Utah. "So, when my graduate student showed me pictures of the uniformity of cells from the brain, I was shocked."
"It suggested that there was some very strong reason why only this population of cells were making it that far into the body."
Already aware that the fungus could grow up to 10 times its normal size in the lungs, the scientists set out to try and find out just why cells of a specific size were found so deep in host territory.
Infecting mice with C. neoformans in various sizes, the researchers discovered that it was the smallest cells that tended to find their way into the brain.
That wasn't all though – the team detected changes to the surfaces of the smaller cells and differences in the genes that were active in those fungi. The researchers suggest that these 'seed cells' aren't just shrunk down versions of the fungus, but something quite different.
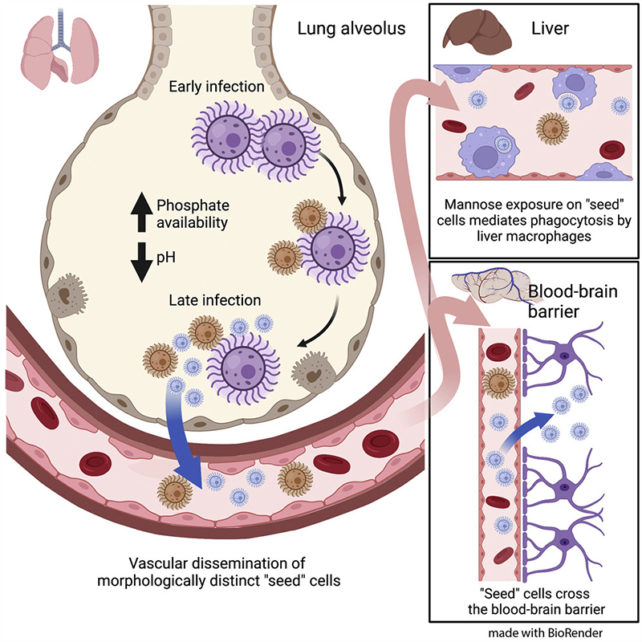
These changes are probably powered by phosphate, based on the scientists' experiments. Not only is phosphate released from host cells when tissues are damaged during infection, the element is plentiful in bird droppings too. This seems to be the catalyst for the fungus being able to shapeshift, which might help it infect their hosts and reach the brain.
"We think that selective pressures from environmental niches like pigeon guano are somehow able to confer to C. neoformans the ability to infect mammals," says Brown.
In the mice experiments, these seed cells were able to make it all the way to the brain in a matter of days. The way in which the fungus is able to adapt so quickly to different environments is key to its success in spreading through the body, the researchers claim.
The next steps are to establish that this same shrinking also happens in humans, and to find drugs that can block this process and prevent C. neoformans from causing damage to the body. The researchers think there might be existing, regulatory approved compounds that could be effective.
Together with recent research into how the fungus breaks through the blood-brain barrier that guards the brain, we're gradually improving our understanding of the tricks that this deadly fungus uses to spread its infection.
"We demonstrate that the formation of a small C. neoformans morphotype – called 'seed' cells due to their colonizing ability – is critical for extrapulmonary organ entry," write the researchers in their published paper.
The research has been published in Cell Host & Microbe.