Many people have never heard of it, but hereditary haemochromatosis is the most common genetic disease in the Western world, with 250,000 people of European ancestry in the UK affected and a million in the US.
The faulty genes responsible cause excessive absorption of iron, which sometimes builds up to toxic levels.
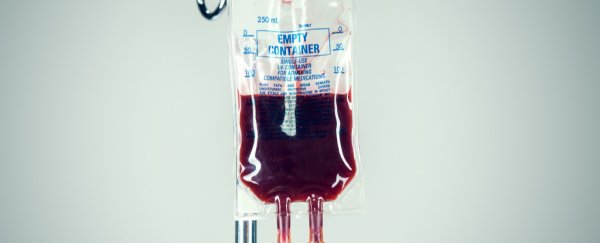
We've now shown that these faulty genes cause more damage around the body than previously thought. But the good news is that the treatment is simple. It involves donating blood to bring iron levels down.
Over the last 15 years, our research group at the University of Exeter has focused on the question: why are some older people ill and frail in their sixties while others remain active and disease free into their nineties and beyond.
In our most recent study, we used data from the UK Biobank, which contains genetic and medical data from half a million people, to find genes associated with muscle ageing, searching across people's DNA.
To our surprise, we found a link between the haemochromatosis gene and muscle weakness, chronic pain and frailty in the older people in the study who weren't diagnosed with haemochromatosis.
UK Biobank studied 500,000 volunteers who were interviewed when they were 40 to 70 years old, and we have data from their hospital records for an average of seven years after the interview.
We were able to study 2,890 people with both faulty haemochromatosis genes (called HFE C282Y mutations), making the study nearly ten times larger than any previous similar one.
Severe consequences if not treated early
Our papers, in the BMJ and the Journal of Gerontology: Medical Sciences, report that those with the two faulty genes have quadruple the rates of liver disease and double the rates of arthritis and frailty compared with the general population.
They also have higher rates of liver cancer, diabetes (both type 1 and type 2), chronic pain and tiredness. Both the younger (40- to 59-year-olds) and the older group (60 to 70) were affected.
The more severe effects of the faulty genes are fairly frequently seen in healthcare. Of all the hip replacements in men in the UK Biobank study, 1.6 percent were in men with the two faulty genes. Nearly 6% of all the liver cancers in the study were also in people with the faulty genes.
Women tend to be diagnosed with haemochromatosis at older ages than men, as they have partial protection from losing iron through menstruation and having children, although some younger women do develop the disease.
Most of the excess liver disease, arthritis, diabetes, tiredness, pain and muscle weakness could be prevented if treatment is started before damage from excess iron sets in.
To maintain low iron levels, people with the two faulty genes need to give blood three or four times a year. The blood can even be used by others for transfusion – a rare win-win.
If the disease is left untreated, very high iron levels can build up causing permanent damage, and blood might have to be taken once every two weeks for a year and destroyed.
Economic models show that routine screening of people of European ancestry for haemochromatosis would more than pay for itself.
The iron absorption mutation may have become common when hunter-gatherers switched to agriculture in low iron areas, over 10,000 years ago.
Between 10 and 15 percent of people with northern European ancestry carry one copy of the C282Y mutation, with about one in 150 inheriting the high-risk two copies. People of southern European ancestry have about half that rate of the faulty genes.
More screening is needed
Symptoms of haemochromatosis are not easy to diagnose without specific blood or genetic testing. The joint pains and arthritis in haemochromatosis develop in a similar way to osteoarthritis, with the differences between the two being difficult to spot.
Of course, doctors see many patients with tiredness, most of whom don't have haemochromatosis.
The joint pains and tiredness are sometimes mistaken as "normal" signs of ageing by both patients and doctors. It is only when the more severe damage is done to the liver that the disease becomes easier to recognise.
It is clear that routine testing is needed if patients are to be identified early enough, and it is exciting to think that such a large amount of disease could be avoided by such a simple treatment. The prospect of halving frailty rates in older people with these faulty genes is also very exciting. ![]()
Janice Atkins, Research Fellow, University of Exeter; David Melzer, Professor of Epidemiology and Public Health, University of Exeter, and Luke Pilling, Research Fellow in Genomic Epidemiology, University of Exeter.
This article is republished from The Conversation under a Creative Commons license. Read the original article.