Down on an atomic level, glass is a jumbled mess of atoms, which makes it easily prone to distortion and cracking. Now, chemists have discovered how to arrange the atoms within glass in such a way, the resulting material can even rival the strength of diamonds.
A team of materials scientists from Yanshan University in China has discovered the critical proportion of crystallized and amorphous carbon needed to create a glass with remarkable properties that won't weaken under intense pressure.
The mechanical properties of a material often come down to the way its building blocks link together. Diamond's notorious toughness is determined by the four bonds every single one of its carbon atoms makes with its neighbors. Though these bonds make for a solid bridge, they also don't leave any electrons spare to carry a current, effectively making diamond an insulator.
Glassy solids don't have repeating patterns, at least on a general scale. Their overall structure is more or less what you get when a liquid's particles all drop in place once the temperature drops low enough.
Depending on the constituent ingredients, however, glassy materials can have a surprising degree of structure up close. Their disordered arrangement also allows for a wide range of optical and mechanical properties that makes them better suited for certain technologies.
Glasses that are based on metals should combine the advantages of both, lending a degree of strength that crystalline metals don't have, while still being conductive.
Determining what a glassy state of carbon might behave like, however, is tricky to predict based on theory alone.
So the Yanshan researchers experimented, squishing spheres of carbon atoms called 'buckyballs' under intense pressure of around 25 gigapascals (just under 250,000 atmospheres) and then baking the mush at temperatures between 1,000 and 1,200 degrees Celsius (about 1,800 to 2,200 degrees Fahrenheit).
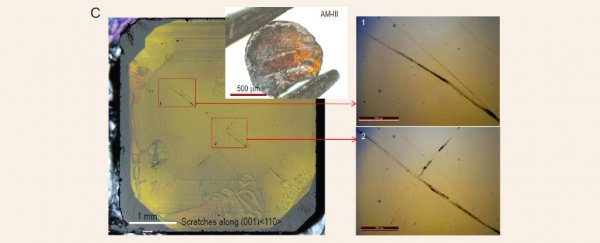
Subjecting the products, dubbed AM-I, II, and III, to a litany of tests, the chemists mapped the way the atoms bonded with one another, showing they all operated as a semiconductor on a level comparable with amorphous silicon.
But it was the mechanical properties of the third result that truly stood out.
Diamond is characteristically known as one of the hardest known substances. A common measure of hardness, called the Vickers hardness test, actually uses a diamond tip to indent material. The harder the material, the greater the force (measured in gigapascals) required to leave a sizable mark.
Scratching another diamond might require somewhere between 60 and 100 gigapascals, depending on whether it's natural or made with care in a lab.
The glassy material AM-III measured somewhere between 110 and 116 gigapascals on the Vickers hardness test, making it the hardest amorphous solid to date. Running a slither of the substance along the flat face of a natural diamond left a clear score line.
Producing enough of the material to use widely in commercial processes would be an expense few would fork out on right now. In time, enough might be made to serve as a replacement for silicon transistors used in high-pressure environments.
Given how experimental this glass development was, it's possible there's more to be found by squashing and cooking other carbon allotropes, such as graphene, at a range of pressures and temperatures.
Materials science is well into the carbon age of late, coming up with ingenious new ways to put mechanical and electrical properties of variously-arranged carbon atoms to work.
How we'll use AM-III is hard to say right now, but one day it just might become an electrical engineer's best friend.
This research was published in National Science Review.