All life is made up of cells several magnitudes smaller than a grain of salt. Their seemingly simple-looking structures mask the intricate and complex molecular activity that enables them to carry out the functions that sustain life.
Researchers are beginning to be able to visualize this activity to a level of detail they haven't been able to before.
Biological structures can be visualized by either starting at the level of the whole organism and working down, or starting at the level of single atoms and working up.
However, there has been a resolution gap between a cell's smallest structures, such as the cytoskeleton that supports the cell's shape, and its largest structures, such as the ribosomes that make proteins in cells.
By analogy of Google Maps, while scientists have been able to see entire cities and individual houses, they did not have the tools to see how the houses came together to make up neighborhoods.
Seeing these neighborhood-level details is essential to being able to understand how individual components work together in the environment of a cell.
New tools are steadily bridging this gap. And ongoing development of one particular technique, cryo-electron tomography, or cryo-ET, has the potential to deepen how researchers study and understand how cells function in health and disease.
As the former editor-in-chief of Science magazine and as a researcher who has studied hard-to-visualize large protein structures for decades, I have witnessed astounding progress in the development of tools that can determine biological structures in detail.
Just as it becomes easier to understand how complicated systems work when you know what they look like, understanding how biological structures fit together in a cell is key to understanding how organisms function.

A brief history of microscopy
In the 17th century, light microscopy first revealed the existence of cells. In the 20th century, electron microscopy offered even greater detail, revealing the elaborate structures within cells, including organelles like the endoplasmic reticulum, a complex network of membranes that play key roles in protein synthesis and transport.
From the 1940s to 1960s, biochemists worked to separate cells into their molecular components and learn how to determine the 3D structures of proteins and other macromolecules at or near atomic resolution. This was first done using X-ray crystallography to visualize the structure of myoglobin, a protein that supplies oxygen to muscles.
Over the past decade, techniques based on nuclear magnetic resonance, which produces images based on how atoms interact in a magnetic field, and cryo-electron microscopy have rapidly increased the number and complexity of the structures scientists can visualize.
What is cryo-EM and cryo-ET?
Cryo-electron microscopy, or cryo-EM, uses a camera to detect how a beam of electrons is deflected as the electrons pass through a sample to visualize structures at the molecular level.
Samples are rapidly frozen to protect them from radiation damage. Detailed models of the structure of interest are made by taking multiple images of individual molecules and averaging them into a 3D structure.
Cryo-ET shares similar components with cryo-EM but uses different methods. Because most cells are too thick to be imaged clearly, a region of interest in a cell is first thinned by using an ion beam.
The sample is then tilted to take multiple pictures of it at different angles, analogous to a CT scan of a body part – although in this case the imaging system itself is tilted, rather than the patient. These images are then combined by a computer to produce a 3D image of a portion of the cell.
The resolution of this image is high enough that researchers – or computer programs – can identify the individual components of different structures in a cell. Researchers have used this approach, for example, to show how proteins move and are degraded inside an algal cell.
Many of the steps researchers once had to do manually to determine the structures of cells are becoming automated, allowing scientists to identify new structures at vastly higher speeds.
For example, combining cryo-EM with artificial intelligence programs like AlphaFold can facilitate image interpretation by predicting protein structures that have not yet been characterized.
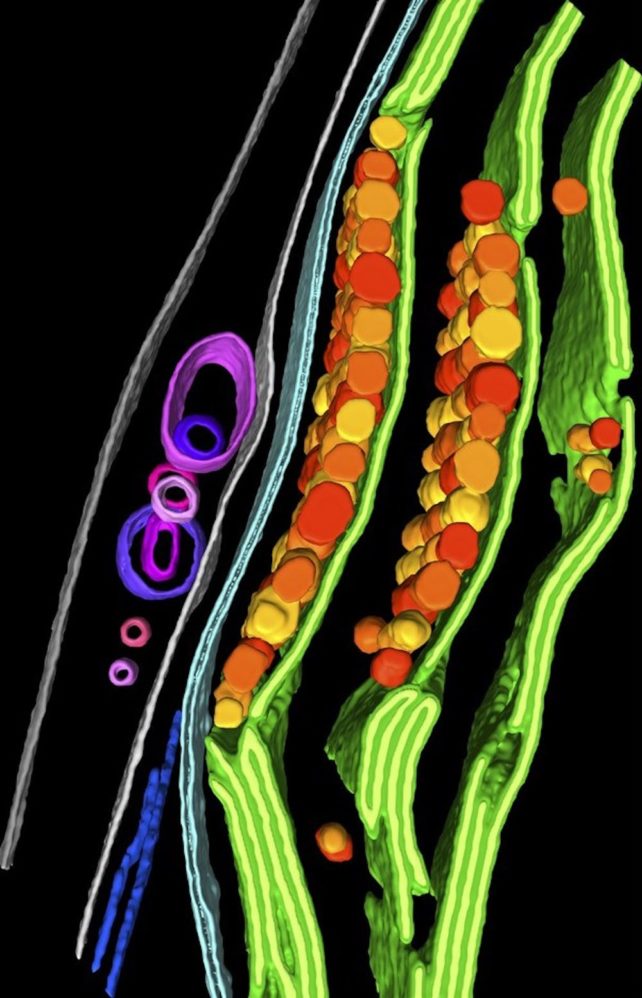
Understanding cell structure and function
As imaging methods and workflows improve, researchers will be able to tackle some key questions in cell biology with different strategies.
The first step is to decide what cells and which regions within those cells to study. Another visualization technique called correlated light and electron microscopy, or CLEM, uses fluorescent tags to help locate regions where interesting processes are taking place in living cells.
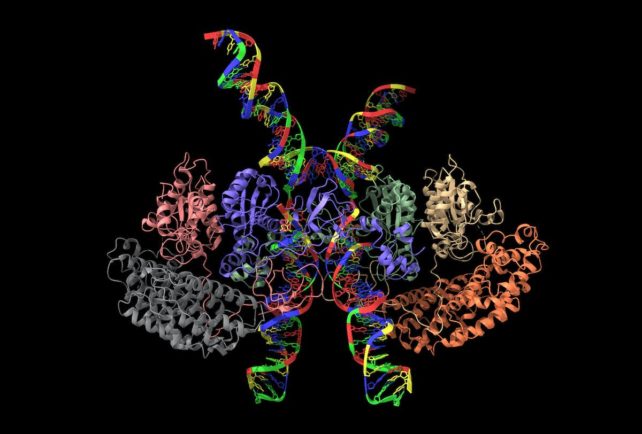
Comparing the genetic difference between cells can provide additional insight. Scientists can look at cells that are unable to carry out particular functions and see how this is reflected in their structure. This approach can also help researchers study how cells interact with each other.
Cryo-ET is likely to remain a specialized tool for some time. But further technological developments and increasing accessibility will allow the scientific community to examine the link between cellular structure and function at previously inaccessible levels of detail.
I anticipate seeing new theories on how we understand cells, moving from disorganized bags of molecules to intricately organized and dynamic systems.![]()
Jeremy Berg, Professor of Computational and Systems Biology, Associate Senior Vice Chancellor for Science Strategy and Planning, University of Pittsburgh
This article is republished from The Conversation under a Creative Commons license. Read the original article.
