Prompted by all the comments telling him he's going to die a slow and horrible death every time he deals with molten metals, YouTube's The Backyard Scientist decided to melt down a whole lot of different types of metal and toss them into a 190-litre tank of water to see what happens. He does it so we don't have to source all that tin, pewter, bismuth, lead, zinc, aluminium, and thermite, and ruin all our pots and pans melting them down in an effort to see how cool they look when reacting with water.
The metals used in the video go in order from lowest melting point to highest, and it sure does get high at the upper end there:
Pewter: 170–230°C (338–446°F)
Tin: 231.9°C (449.5°F)
Bismuth: 271.4°C (520.6°F)
Lead: 327.5°C (621.5°F)
Zinc: 419.5°C (787.2°F)
Aluminium: 660.3°C (1,221°F)
Iron: 1,538°C (2,800°F) Reaction: 2200°C (4000°F)
First up is pewter, and this light metal alloy - made up of mostly tin - erupts instantaneously into little gas explosions as soon as it hits the water. Meanwhile, lead forms an outer coating to prevent explosions, and forms neat little spears that delicately jab the bottom of the tank, tin turns into popcorn, and all that beautiful bismuth just disintegrates into dust.
With a melting point of 419.5°C, zinc is a bit more heavy-duty than the previous metals, and even started to sweat as it was melted down. "I've never seen anything like that happen on metal before," says The Backyard Scientist in the video above. And wait till you see its reaction to water. With some amazing "pew pew!" sounds, the zinc plummets through the tank like little metal raindrops, almost entirely unaffected by the water.
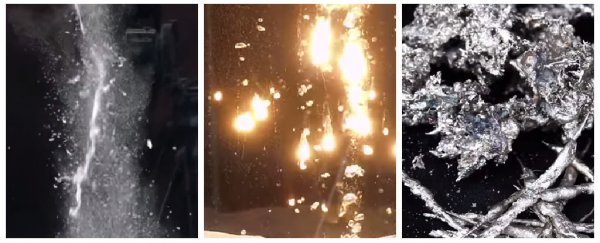
Next up is a joke about the correct spelling of aluminium at the expense of everyone in the world who doesn't live in America, and some more amazing pew pews as this common metal snakes its way through the tank. Thanks to its super-high melting point, it remains molten all the way down to the bottom.
And now it's time to get really, really weird. Thermite doesn't really look like a metal - more like some fine, red powder pretending to be a candle. This pyrotechnic mixture needs to be heated up to a whopping 1,538°C to produce molten iron. I'm not going to spoil what happens, but let's say there's going to be fireworks… Watch the video above to see the sparkles in all their golden glory.
H/T: Digg