Scientists have identified a key molecule exploited by HIV when the virus infects human cells, and the discovery could represent a major step forward in defeating the deadly disease.
Using a new kind of microscopy technique, researchers isolated a small molecule called inositol hexakisphosphate (IP6). They think the virus might hijack this molecule in host cells, using it to both shield itself from our immune system, and release its viral payload.
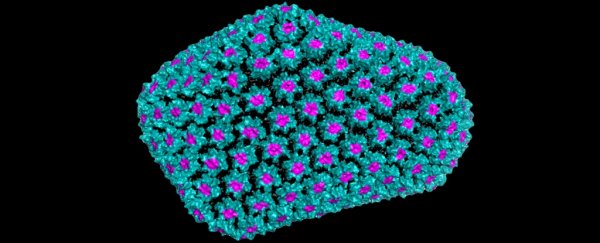
The genetic material of viruses like HIV is housed within a protective protein coating called a capsid, which not only helps safeguard the virus from a host's defence mechanisms, but enables it to deliver its viral contents to infected cells.
Despite decades of research, though, there's still a lot we don't know about how HIV functions in this regard, but with the identification of the potential role of IP6 – which the virus could be 'recruiting' from host cells to bolster its capsid – the mysteries are closer than ever to being solved.
"For as long as it has been studied, the HIV capsid has been known to be highly unstable on its own," structural virologist David Jacques from UNSW in Australia explained to ScienceAlert.
"This has led to theories that maybe its lack of stability is somehow important to infection. With our discovery of IP6, we now know that during infections the HIV capsid is never 'on its own'. It is always exposed to IP6, which dramatically stabilises the core of the virus."
Scientists have known for decades that the IP6 molecule was capable of helping assemble viral components into virions (complete virus particles), but it was unclear how important the molecule was in HIV's overall life cycle.
Part of the problem, according to Jacques, is the intrinsic instability of the capsids – which quickly collapse when they're extracted from virions, making them difficult to study in the lab.
The new research shows why the capsid container crumbles so easily.
"We now know that the problem was a missing ingredient: IP6," says Jacques.
Just as the hijacked compound enables HIV to fortify its capsid – stabilising the structure for up to 20 hours – once this defensive glue is removed at a molecular level, the capsid readily breaks down.
"Now that we know that IP6 is always present during normal infections, we can add this compound to stabilise the capsid in the test tube," Jacques explains.
"This opens up whole new avenues of research focused on understanding how the capsid works, because we now have time to study it."
The discoveries – made possible by a new microscopy technique that uses fluorescence to monitor capsid breakdowns in real-time – can measure the stability of thousands of individual virions (including immature ones) to determine what specific effect compounds like IP6 are having on them.
"We speculate that the immature virus collects IP6 when it leaves the producer cell (where the virus is made), and then uses it again after entering the target cell to stabilise its capsid and facilitate infection," lead researcher and UNSW molecular scientist Till Böcking explained to ScienceAlert.
The team's method showed IP6 binds to pores on the viral capsid, with the strengthened coating enabling a greater-than-100-fold increase in the accumulation of new viral DNA inside the structure.
That's not an insignificant boost for something as deadly as HIV, and now that we know how effective this hijacked molecule is for the virus's infectivity, we might actually be able to exploit IP6 ourselves: as a new target for future antiviral treatments.
"Once we understand the molecular details of these processes, we could devise strategies to 'trick' the virus into releasing IP6 prematurely," says Böcking.
"Or locking it in, such that it can no longer control the correct timing for capsid uncoating."